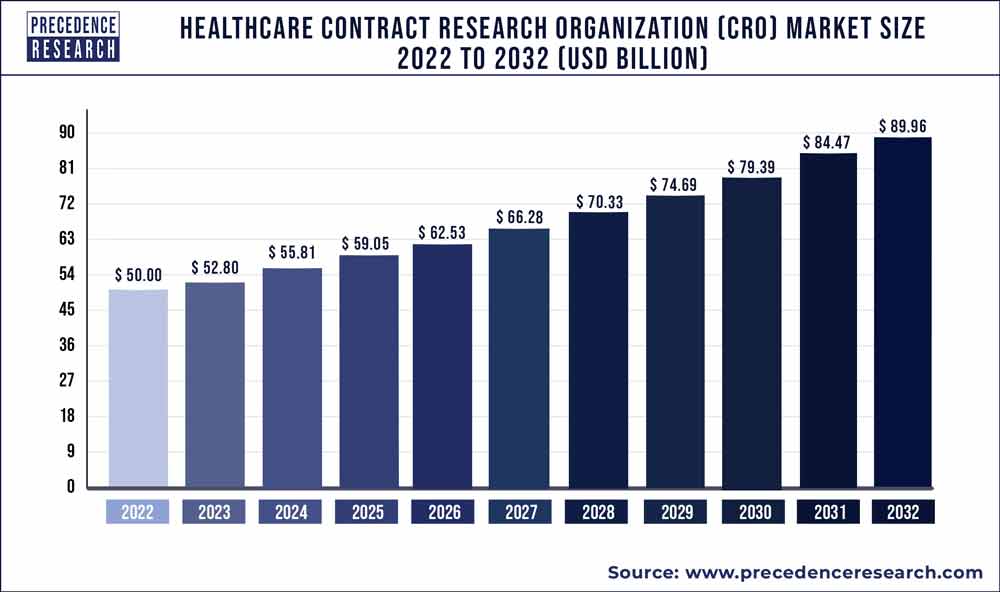
The global healthcare contract research organization market was valued at USD 50 billion in 2022 and is expected to hit over USD 89.96 billion by 2032, expanding growth at a CAGR of 6.10% from 2023 to 2032.
The rising prevalence of various chronic diseases and rapidly growing geriatric population is boosting the demand for the various new and innovative life-saving drugs across the globe. Thus, the rising burden of diseases is fueling the investments by the top pharmaceutical companies in the research & development of various drugs and diagnostic devices, which is driving the growth of the healthcare CRO market.
Get the Sample Pages of Report for More Understanding@ https://www.precedenceresearch.com/sample/1538
The specialized research services along with various other services offered by the healthcare CROs such as data management, clinical supply management/project management, medical writing, clinical monitoring, patient & site recruitment, bio-statistics, and regulatory & medical affairs is boosting the demand for the healthcare CRO services. The outsourcing of activities helps the clients to save time and costs, which is a major factor that drive the healthcare CRO market across the globe. During the pandemic in 2020, the healthcare CRO market has witnessed a rapid surge in the revenues as the top pharma companies were heavily investing the research and development of the drugs and vaccines for the COVID-19 disease.
Report Highlights
- Based on the service, the clinical monitoring segment dominated the global healthcare CRO market in 2020. The presence of specialized and experienced scientists in the CROs and the adoption of advanced and smart analytical tools in the CROs helps to provide specialized and efficient services at a low cost. This is a major factor that drives the growth of the clinical monitoring segment.
- Based on the type, the clinical segment accounted for a market share of around 75% and dominated the market in 2020. This is because a huge cost is involved in the Phase III of the clinical trials, which generates a huge revenue for the healthcare CRO market across the globe.
Regional Snapshot
North America dominated the global healthcare contract research organization market in 2020. The presence of top pharmaceutical companies along with the growing number of CROs in the region has significant contributions in the growth of the market. According to the Pharmaceutical Research and Manufacturers Association (PhRMA), the maximum research and developmental activities occur in US and US also holds the patent and intellectual property rights of the majority of the new developed drugs. The healthcare CRO market is supported by the favorable government regulations and the active participation of the US FDA in the clinical trials and approvals has exponentially driven the growth of the North America healthcare CRO market in the past decade.
On the other hand, Asia Pacific is estimated to be the most opportunistic market during the forecast period. Asia Pacific is known due to the presence of several top healthcare CROs in the countries like India, China, and South Korea. The availability of expert research professionals at cheap costs attracts the pharmaceutical companies to invest in the region. Moreover, the rising burden of diseases is fueling the demand for the drug discovery services of the CROs in the region, which is further expected to drive the growth of the Asia Pacific healthcare CRO market in the forthcoming years.
Scope of the Healthcare Contract Research Organization (CRO) Market
| Report Coverage | Details |
| Market Size | US$ 89.96 Billion by 2032 |
| Growth Rate | CAGR of 6.10% from 2023 to 2032 |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | Service, Type, Region |
Healthcare Contract Research Organization (CRO) Market Dynamics
Driver
Growing investments in the pharmaceutical research and development activities
Majority of the biopharmaceutical, biosimilar, and medical device companies are investing heavily in the research & development of innovative drugs and medical devices across the globe. The research and development intensive pharmaceutical industry, is heavily investing for the clinical trials, drug discovery, data management, and various other services offered by the CROs to develop new drugs. It is estimated that the global pharmaceutical research and development expenditure increased from around US$135 billion in 2012 to US$185 billion in 2019. Therefore, the increasing investments by the pharmaceutical companies is the most prominent driver of the global healthcare CRO market.
Restraint
Demand for faster results may hamper the research quality
The healthcare CROs faces extensive pressure to produce desired results from their clients. The costly the research projects and activities may loss its credibility due to the increased pressure of producing results in a short span of time. This may hamper the growth of the market during the forecast period.
Opportunity
Rapidly growing biologics industry
The development of innovative drugs for curing various non-communicable diseases in the biologic industry has gained rapid traction. The rising prevalence of the chronic diseases across the globe is offering the biologics industry a lucrative growth opportunity, which compels them to increasing invest in the research activities and outsource these activities to the specialized healthcare CROs. This is creating a huge growth opportunity for the healthcare CRO market players.
Challenge
Lack of skilled and experienced professionals
The healthcare CRO industry faces a shortage of skilled and experienced professionals. The recruitment and retention of the skilled scientists in a contract research organization is a major challenge faced by the healthcare CRO market players that has a negative impact on the market.
Read Also: AI in Pharmaceutical Market Size, Share, Report By 2032
Some of the prominent players in the market include:
- QVIA
- LabCorp
- Charles River Laboratories
- WuXiAppTec
- Syneos Health
- Parexel International
- PPD
- ICON Plc
- Medpace Holdings
- SGS
- PSI CRO AG
- Axcent Advanced Analytics
- BIO Agile Therapeutics
- Firma Clinical Research
- Acculab Life Sciences
Segments Covered in the Report
By Service
- Data Management
- Clinical Supply Management/Project Management
- Medical Writing
- Clinical Monitoring
- Patient & Site Recruitment
- Bio-Statistics
- Regulatory & Medical Affairs
- Investigator Payments
- Technology
- Laboratory
- Quality Management
- Others
By Type
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
By Application
- Oncology
- Cardiovascular
- Autoimmune/Inflammation
- Central nervous system (CNS)
- Dermatology
- Infectious diseases
- Diabetes
- Pain
- Others
By End-use
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Others
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of the Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/
- Orthopedic Implants Market Size, Share, Share, Report 2032 - April 25, 2024
- Advanced Drug Delivery Market Size, Trends, Report By 2032 - April 25, 2024
- Artificial Intelligence (AI) In Drug Discovery Market Size Report by 2032 - April 25, 2024