Table of Contents
ToggleUnderstanding the Clinical Trial Materials Manufacturing Market
The clinical trial materials manufacturing market has become increasingly essential to the success of global drug development. As clinical trials grow more complex and globally distributed, the ability to manufacture and distribute trial materials efficiently has become a top priority for sponsors and CROs alike.
These materials include investigational products, placebos, comparator drugs, as well as secondary items such as syringes, vials, and labeling kits. A well-coordinated manufacturing and logistics operation ensures that clinical supplies reach study sites and patients in a timely and compliant manner, directly influencing trial timelines and costs.
AI and Technological Advancements in Manufacturing
AI is playing a major role in streamlining the clinical trial materials manufacturing market. Forecasting algorithms now predict material demand across various regions, while intelligent inventory management systems reduce waste and prevent overstocking.
Additionally, machine learning is improving batch consistency and quality assurance, especially for temperature-sensitive biologics. Automated packaging and labeling processes are minimizing human error, while AI-enabled audits and compliance checks are reducing risk and ensuring adherence to regulatory standards.
Future Trends in the Market
Decentralized trials are reshaping how the clinical trial materials manufacturing market operates. Direct-to-patient supply models, remote monitoring tools, and real-time inventory tracking are now essential components of a responsive supply chain.
Emerging technologies such as 3D-printed drug products, smart packaging with integrated sensors, and digital serialization are expected to become standard. There is also a strong shift toward sustainability in manufacturing, with many sponsors prioritizing eco-friendly materials and carbon-neutral logistics strategies.
Rising Demand and Complexity of Clinical Trials
The rise in rare disease studies, personalized medicine, and global trials has driven demand within the clinical trial materials manufacturing market. Trial sponsors must accommodate highly specific formulations, tight delivery windows, and the ability to pivot quickly if study protocols change.
Furthermore, the increase in combination therapies and advanced biologics means that materials manufacturing must keep pace with the evolving science. This growing demand has created opportunities for innovative suppliers capable of scaling rapidly without compromising compliance.
Key Market Highlights
Over the last few years, the clinical trial materials manufacturing market has witnessed considerable innovation and investment. Hybrid manufacturing models that integrate physical and digital workflows are on the rise, enabling real-time status updates and improved visibility for sponsors.
Cold chain infrastructure improvements have expanded the market’s ability to handle complex biologics and cell therapies. Moreover, more partnerships between CMOs and digital health platforms are emerging to synchronize production and logistics with eClinical tools.
Growth Drivers of the Market
Several forces are accelerating the clinical trial materials manufacturing market. The overall increase in clinical trials, regulatory encouragement for faster drug development, and technological maturity all serve as strong tailwinds.
Outsourcing trends continue to grow, as sponsors seek flexible partners who can respond to fluctuations in trial design or volume. Additionally, the growth of clinical research in emerging economies has expanded the demand for compliant, localized material production.
Challenges and Restraints in the Market
Despite its strong outlook, the clinical trial materials manufacturing market faces challenges, including fluctuating raw material costs and global supply chain instability. Ensuring product integrity during long-distance shipping—especially for temperature-sensitive items—requires highly reliable infrastructure.
Compliance remains a top concern, particularly in multinational trials with varying regulatory frameworks. Manufacturing timelines can be delayed by quality issues, customs hold-ups, or changes in study protocol, placing additional strain on trial sponsors.
Opportunities for Innovation and Expansion
The clinical trial materials manufacturing market is rich with opportunities for innovation, particularly as demand shifts toward agility and precision in manufacturing. One of the most promising avenues is the integration of cloud-based manufacturing management systems. These platforms allow real-time tracking of supplies, automated quality assurance, and predictive maintenance, ultimately reducing delays and improving cost efficiency.
Emerging biopharma companies often seek scalable partners to support rapid trial expansion. This opens doors for small-to-midsize CMOs offering niche, patient-specific formulations or specialty packaging. Furthermore, innovations like temperature-controlled smart containers with live tracking, blockchain for secure chain-of-custody, and digital twins to simulate manufacturing workflows present a frontier for market differentiation.
Geographical expansion is another key opportunity. Companies that invest in establishing regional GMP-compliant manufacturing hubs in fast-growing clinical research markets—like Southeast Asia, Eastern Europe, and parts of Africa—stand to gain a competitive edge. As trials become more globally distributed, proximity to patient populations will become a strategic advantage.
Regional Insights
The clinical trial materials manufacturing market displays varying dynamics across regions. North America continues to dominate in terms of capacity and technological advancement. The U.S. leads globally in clinical trial activity, with robust infrastructure and significant investment in manufacturing innovation. Canada’s growing biotech sector and regulatory efficiency also contribute to the region’s strength.
Europe maintains its leadership position with well-established CMOs in countries like Germany, the Netherlands, and Switzerland. The region is known for its compliance with EU regulations and its high-quality output, making it a trusted destination for global sponsors. Brexit has created some operational shifts, but the UK still holds considerable manufacturing capabilities.
Asia-Pacific is rapidly emerging as a hotspot for clinical research and material manufacturing. India and China are experiencing accelerated growth due to cost advantages, regulatory reforms, and government incentives. Japan and South Korea also contribute significantly, with advanced infrastructure and strong pharmaceutical industries. These regions are expected to be focal points of outsourcing growth in the next decade.
Latin America is gaining traction as a strategic region due to patient diversity and lower operational costs. Brazil, Mexico, and Argentina are key players, with increasing local production capabilities. Meanwhile, Middle East and Africa, though currently limited in infrastructure, are beginning to attract investment. Local governments are initiating frameworks to support clinical research, creating long-term potential for manufacturing development.
Also Read@ https://www.pharma-geek.com/dehydration-monitoring-devices-market/
Wearable Biometric Monitor Market Companies

- Abbott (FreeStyle Libre wearable tech)
- ActiGraph
- Apple Inc.
- BioIntelliSense
- Biostrap
- Cardiac Insight
- Empatica
- Fitbit (Google)
- Garmin Ltd.
- Huawei Technologies
- Oura Health (Oura Ring)
- Philips Healthcare
- Polar Electro
- Samsung Electronics
- Valencell
- VitalConnect
- Whoop Inc.
- Withings
- Xiaomi Corporation
- Zephyr Technology (Medtronic)
Get Free Sample Link @ https://www.precedenceresearch.com/sample/6684
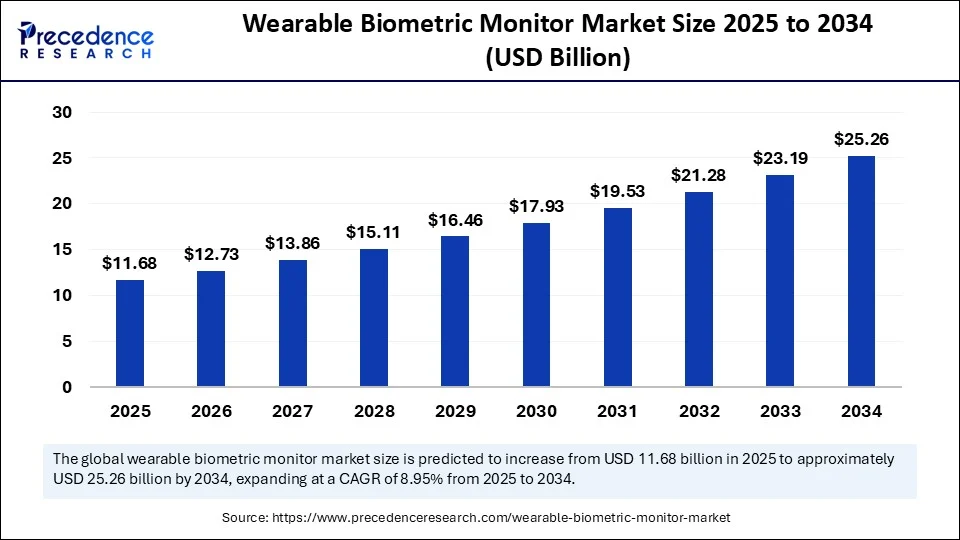
- Wearable Biometric Monitor Market Size to Reach USD 25.26 Billion by 2034 - September 3, 2025
- Clinical Trial Materials Manufacturing Market Size, Report by 2034 - September 3, 2025
- Predictive Genetic Counselling Market Size to Reach USD 8.43 Billion by 2034 - September 3, 2025
