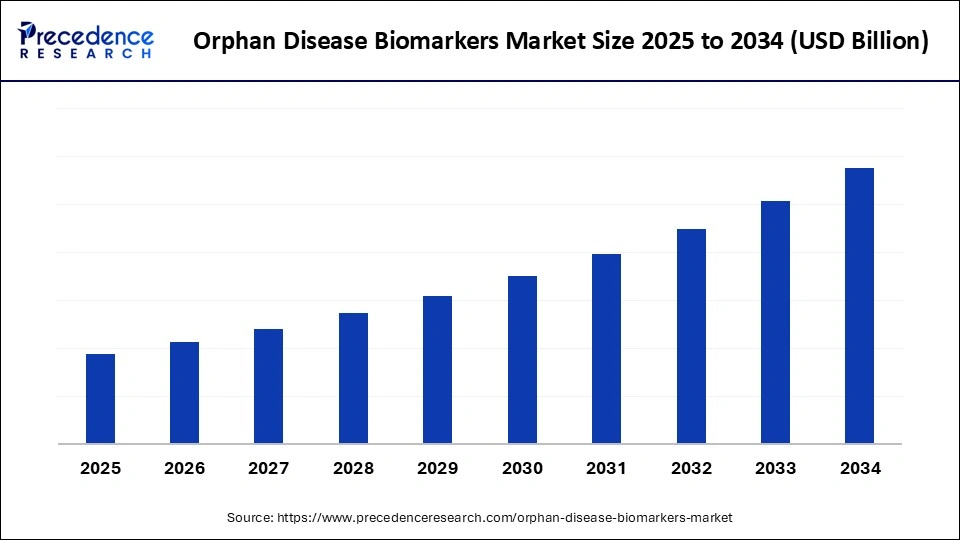
1. Market Overview
The Orphan Disease Biomarkers Market is becoming a strategic priority in the biopharmaceutical landscape. As healthcare shifts toward personalized and rare disease management, biomarkers are taking center stage in diagnostics and drug development.
The market is witnessing an influx of investment from pharma companies and startups alike. Growth is fueled by increased demand for precision medicine, high regulatory interest, and improved infrastructure to support rare disease research. Stakeholders see the Orphan Disease Biomarkers Market as an untapped frontier with long-term value.
2. AI and Innovation in the Market
Innovation defines the competitive edge in the Orphan Disease Biomarkers Market. Companies are turning to AI-driven bioinformatics to unlock new biomarkers faster and with greater accuracy. Machine learning models are helping uncover previously undetectable patterns in rare disease data.
Startups specializing in digital pathology, image-based biomarker analysis, and deep phenotyping are disrupting traditional discovery methods. Innovation hubs around the world are collaborating to develop platforms that combine clinical, genetic, and environmental data to fuel the next generation of biomarker breakthroughs.
3. Future Trends in the Market
The Orphan Disease Biomarkers Market is heading toward a future of platform-based biomarker discovery. Companies are investing in cloud-based biomarker databases, real-time analytics tools, and AI-integrated clinical trial software.
We can also expect broader adoption of decentralized diagnostics and point-of-care testing. New reimbursement models that support early biomarker-based screening in rare diseases will likely emerge. The integration of blockchain for data security in rare patient populations is another developing trend.
4. Rising Demands of the Market
Demand in the Orphan Disease Biomarkers Market is growing across the board. Biopharma firms require biomarker strategies to meet regulatory approval. Hospitals and diagnostic labs need reliable tools for early diagnosis. Patients and advocacy groups push for faster identification and personalized care options.
With each rare condition posing unique diagnostic challenges, the need for disease-specific biomarkers has never been higher. This rising demand is catalyzing M&A activity, licensing deals, and partnership announcements across the diagnostics value chain.
5. Key Market Highlights
-
Investment in rare disease diagnostics reached new highs post-pandemic.
-
AI-powered platforms outperform traditional methods in speed and scalability.
-
Biomarkers are becoming essential for rare disease drug approvals.
-
Academic-industry collaborations are fueling innovation pipelines.
-
Asia-Pacific emerges as a critical manufacturing and R&D hub.
6. Market Growth Drivers
The key drivers accelerating the Orphan Disease Biomarkers Market include:
-
Policy Support: Governments offer fast-track designations and grants.
-
Industry Investment: Big pharma is entering niche diagnostics markets.
-
Technological Maturity: Availability of cost-effective next-gen sequencing.
-
Global Awareness: Rare disease education campaigns are on the rise.
-
Strategic Alliances: Biotech-CDMO partnerships for biomarker development.
These drivers collectively ensure a strong upward trend for the industry.
7. Market Restraints
However, several constraints slow down adoption:
-
Data Fragmentation: Inconsistent data makes cross-condition research difficult.
-
Scalability Issues: Hard to scale platforms for ultra-rare conditions.
-
High Entry Barriers: Regulatory, technological, and financial hurdles for new entrants.
-
Lack of Standardization: Variability in biomarker validation protocols.
Overcoming these barriers is critical to unlocking full market potential.
8. Market Opportunities
Opportunities abound for both incumbents and new players:
-
Digital Biomarkers: Remote disease tracking via mobile platforms.
-
Rare Cancer Biomarkers: High demand for diagnostic companion tools.
-
Global Expansion: Market entry into developing countries with rising healthcare investment.
-
Patient Registries: Biomarker data integration with real-world evidence platforms.
These opportunities position the Orphan Disease Biomarkers Market as a long-term growth area for innovation and commercialization.
9. Regional Insights
-
North America leads in innovation, funding, and rare disease policy enforcement.
-
Europe maintains strong market momentum with pan-European health programs.
-
Asia-Pacific grows rapidly, particularly in precision diagnostics manufacturing.
Orphan Disease Biomarkers Market Companies

- Roche
- Thermo Fisher Scientific
- Abbott Laboratories
- QIAGEN
- Bio-Rad Laboratories
- PerkinElmer
- Illumina
- Agilent Technologies
- Merck KGaA
- Siemens Healthineers
- GE Healthcare
- Danaher Corporation
- Novartis
- BioMérieux
- Abbott Diagnostics
- Medtronic
- F. Hoffmann-La Roche AG
- Labcorp Drug Development
- Invitae Corporation
- Eurofins Scientific
Recent Developments
- In July 2025, NIH issued a Request for Applications (RFA) calling for biomarker and clinical outcome assessment development for rare diseases, to be conducted alongside prospective natural history studies—supporting rigorous trial design.
- In July 2025, Alterity Therapeutics reported positive topline results from its Phase 2 trial of ATH434 in Multiple System Atrophy (MSA), and earlier in May secured Fast Track Designation for the same asset—indicating strong clinical momentum.
- In April 2025, U.S. FDA Commissioner Marty Makary announced plans for a new ultrarare drug approval pathway based on a “plausible mechanism,” potentially enabling conditional approvals without randomized, controlled trials
Get Free Sample Link @ https://www.precedenceresearch.com/sample/6756
- Orphan Disease Biomarkers Market Size, Report by 2034 - September 16, 2025
- LNP CDMO Market Size to Reach USD 820.46 Million by 2034 - September 16, 2025
- Biomarker Discovery Outsourcing Services Market Size to Reach USD 86.74 Billion by 2034 - September 12, 2025
