Neutralizing Antibody Market Key Points
- North America dominated the neutralizing antibody market in 2024.
- Asia Pacific is anticipated to grow at the fastest rate in the market during the forecast period.
- By antibody type, the monoclonal antibodies segment held a significant share of the market in 2024.
- By antibody type, the polyclonal antibodies segment is observed to be the fastest-growing segment during the forecast period.
- By target virus, the influenza segment dominated the market with the highest share in 2024.
- By target virus, the SARS-CoV-2 (COVID-19) segment is seen to grow at the fastest rate in the upcoming period.
- By application, the therapeutics segment held the dominant share of the market in 2024.
- By application, the research & development segment projected to expand rapidly in the coming years.
- By end user, the hospitals & clinics segment led the market in 2024.
- By end user, the research institutes segment is expected to witness the fastest growth during the projected timeframe.
Market Scope
| Report Coverage | Details |
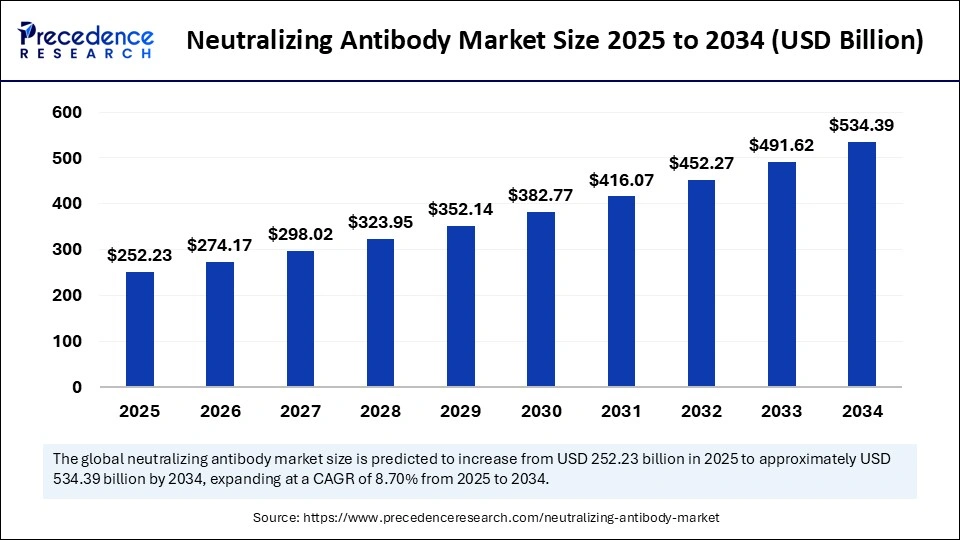
| Market Size by 2034 | USD 534.39 Billion |
| Market Size in 2025 | USD 252.23 Billion |
| Market Size in 2024 | USD 232.04 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.70% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Antibody Type, Target Virus, Application, End User and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Overview
The Neutralizing Antibody Market has emerged as a pivotal segment within the broader biologics and immunotherapy landscape, particularly driven by the need for targeted and efficient therapeutic solutions to combat infectious diseases, autoimmune disorders, and cancer. Neutralizing antibodies (nAbs) are a specialized class of antibodies that can directly inhibit the biological activity of pathogens or disease-associated antigens, such as viruses or mutated proteins, by binding to them with high specificity and preventing their interaction with host cells. Unlike general immunoglobulin treatments, nAbs offer precision therapy by directly interrupting the life cycle of a pathogen or altering disease pathways.
The COVID-19 pandemic acted as a catalyst for accelerated development and commercialization of neutralizing antibody therapies, with several pharmaceutical and biotech firms racing to develop monoclonal antibody-based solutions to mitigate the virus’s spread and impact. This surge highlighted not only the therapeutic potential but also the diagnostic utility of nAbs, propelling further research into their application for a variety of viral infections, including HIV, RSV, hepatitis, and influenza. Additionally, advances in cancer immunotherapy, where checkpoint inhibitors and monoclonal antibodies are being explored for tumor neutralization, have broadened the scope and commercial promise of neutralizing antibodies.
Growth Factors
Several critical factors are contributing to the robust growth of the neutralizing antibody market. One of the primary drivers is the increased prevalence of viral and infectious diseases, which continue to pose major global health threats. Emerging and re-emerging viruses, including SARS-CoV-2, Zika, and Ebola, have underscored the urgency of rapid-response biologics capable of conferring passive immunity or therapeutic relief.
The ongoing innovation in monoclonal antibody (mAb) production technologies—including recombinant DNA technology, phage display, and hybridoma techniques—has drastically improved the yield, safety, and specificity of neutralizing antibodies, making them more accessible and scalable for clinical use.
Another growth factor is the expansion of oncology applications, where nAbs are being developed not only to block oncogenic signaling pathways but also to enhance the body’s immune response against cancer cells. For instance, neutralizing antibodies targeting PD-1/PD-L1 and HER2 are reshaping cancer treatment protocols globally.
Furthermore, increased government funding, public-private partnerships, and regulatory fast-tracking for antibody-based therapies are reducing barriers to clinical development and market entry. The rise in biologics-friendly healthcare policies and insurance coverage, especially in North America and Europe, is further driving adoption.
Impact of AI on the Neutralizing Antibody Market
Artificial Intelligence (AI) is profoundly transforming the neutralizing antibody market by accelerating drug discovery, design, and development cycles. AI-powered platforms are being used to identify novel antigen targets, predict epitope binding sites, and simulate antibody-antigen interactions, significantly reducing the time required to generate effective antibody candidates.
One of the most impactful AI applications is in computational antibody design, where machine learning algorithms can model antibody structures and predict their neutralization efficacy against specific pathogens. This enables a more targeted and efficient screening process, lowering R&D costs and improving hit rates.
AI is also streamlining clinical trial design and patient stratification. By analyzing patient datasets and biomarker profiles, AI tools help identify populations most likely to benefit from nAb therapy, thereby increasing trial success rates and optimizing therapeutic efficacy.
In manufacturing, AI improves process control and quality assurance by monitoring real-time production parameters and predicting potential failures or deviations. This results in more consistent production batches, which is crucial for therapies that must meet stringent regulatory standards.
Additionally, AI is enhancing pharmacovigilance and post-market surveillance, detecting early signs of adverse events or resistance development in patients, and enabling timely therapeutic adjustments or modifications.
Market Drivers
Key drivers propelling the neutralizing antibody market include:
-
Rising incidence of infectious and chronic diseases, necessitating targeted biologic therapies.
-
Proven efficacy and fast-track approvals of neutralizing antibodies during the COVID-19 pandemic, validating their clinical utility.
-
Advances in antibody engineering and molecular biology, allowing for more potent and tailored therapeutics.
-
Expanding applications in oncology and autoimmune diseases, with strong pipeline development by major biotech firms.
-
Growing investments by pharmaceutical companies and research institutions in antibody discovery and development.
Opportunities
The neutralizing antibody market presents significant opportunities for innovation and expansion:
-
Development of broadly neutralizing antibodies (bNAbs) for rapidly mutating viruses like HIV, influenza, and coronaviruses presents a high-impact opportunity.
-
Expansion into low- and middle-income countries (LMICs) through low-cost biosimilar development and technology transfers can unlock untapped market potential.
-
Integration of AI and synthetic biology in antibody design can lead to faster discovery of next-gen nAbs with better pharmacokinetics and fewer side effects.
-
Personalized antibody therapies, tailored to individual patients based on genetic and immunologic profiles, represent the future of precision medicine.
-
Strategic collaborations between academia, startups, and pharmaceutical giants can accelerate development timelines and market reach.
Challenges
Despite its potential, the neutralizing antibody market faces several challenges:
-
High production costs and complex manufacturing processes make biologics expensive, limiting accessibility in certain markets.
-
Cold chain and storage requirements for antibody formulations pose logistical challenges, especially in remote or resource-poor regions.
-
Potential for antibody resistance and escape mutations, particularly in rapidly evolving viruses, can compromise long-term efficacy.
-
Regulatory complexity and stringent quality standards necessitate significant investment in compliance and documentation.
-
Competition from alternative therapies, such as mRNA vaccines and small molecule drugs, may slow market penetration in some disease areas.
Regional Outlook
North America currently leads the neutralizing antibody market, supported by a strong biopharmaceutical industry, well-established regulatory pathways, and high R&D investment. The United States, in particular, witnessed substantial growth during the COVID-19 crisis, with emergency use authorizations for neutralizing antibody cocktails from firms like Regeneron and Eli Lilly.
Europe holds a significant share as well, with countries like Germany, France, and the UK being hubs for biologics research and production. The region’s advanced healthcare infrastructure and robust public funding for medical research support continued market expansion.
Asia-Pacific is expected to witness the fastest growth in the coming years. With the rise of biotech ecosystems in China, India, South Korea, and Japan, the region is not only increasing its domestic production of antibodies but also investing heavily in clinical research. Government initiatives to improve biologics accessibility and establish local manufacturing are key growth enablers.
Latin America, the Middle East, and Africa are emerging regions with growing demand for biologic therapeutics but limited manufacturing capacity. Partnerships with international health organizations and biotech firms are vital for accelerating access to neutralizing antibody therapies in these areas, especially for infectious disease management and public health preparedness.
Neutralizing Antibody Market Companies

- Regeneron Pharmaceuticals, Inc.
- Eli Lilly and Company
- AstraZeneca PLC
- GlaxoSmithKline plc
- Sanofi SA
- Roche Holding AG
- Novartis AG
- Merck & Co., Inc.
- Pfizer Inc.
- Johnson & Johnson
Segments Covered in the Report
By Antibody Type
- Monoclonal Antibodies
- Polyclonal Antibodies
By Target Virus
- SARS-CoV-2 (COVID-19)
- HIV
- Influenza
- Ebola
- Zika
- Hepatitis C
- Others
By Application
- Therapeutics
- Diagnostics
- Research & Development
By End User
- Hospitals & Clinics
- Research Institutes
- Diagnostic Laboratories
- Pharmaceutical Companies
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa (MEA)
Also Read: Amniocentesis Needle Market
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6087
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344
- Arteriovenous Implants Market Enhance dialysis outcomes with advanced grafts, endovascular access, and AI-assisted precision - September 16, 2025
- Smart Retinal Implants Market Restore vision with wireless bioelectronic prosthetics and AI-powered retinal technologies - September 16, 2025
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025
