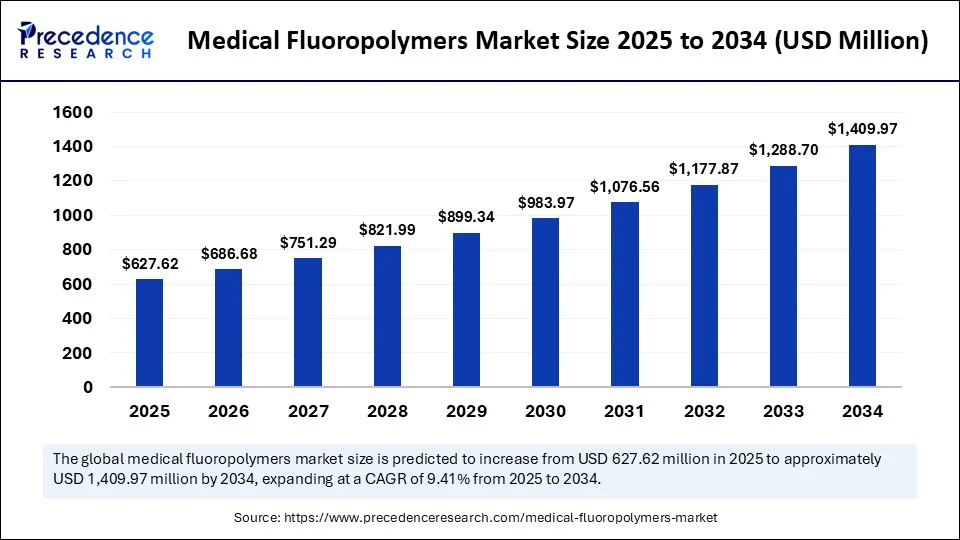
Market Key Takeaways
The Medical Fluoropolymers Market stands out due to its unmatched chemical inertness, exceptional biocompatibility, and superior performance under extreme conditions. Serving critical roles in device manufacturing, pharmaceutical processes, and implantable technologies, it demonstrates consistent global expansion.
Key market takeaways include its strategic concentration in specific applications, high entry barriers due to regulatory and technical complexity, and a competitive landscape where nimble specialty players partner closely with OEMs. Cost-performance balance remains vital: success hinges on delivering technical excellence without excessive cost premium. Overall, the Medical Fluoropolymers Market is marked by measured, technology-driven growth, progressive penetration into emerging geographies, and concentrated application areas demanding high purity and reliability.
Artificial Intelligence of Market
Within the Medical Fluoropolymers Market, “Artificial Intelligence of Market” encompasses the infusion of AI in every critical stage—from upstream R&D to downstream commercialization. AI-powered drug-device predictive models assess material behavior in body environments, anticipating degradation pathways or frictional interactions. In production management, real‑time AI monitoring of temperature, pressure, and polymer fluid dynamics ensures consistent quality and yield.
AI-enhanced demand modeling captures granular shifts—such as seasonal surgery volumes or regional device adoption—boosting forecasting accuracy. Moreover, AI-driven competitor and patent landscape analysis signals where breakthroughs are imminent. This AI sophistication enables faster innovation, tighter process control, smarter commercialization, and more insightful planning across the Medical Fluoropolymers Market.
Market Overview
The Medical Fluoropolymers Market includes a portfolio of high-purity fluoropolymers—PTFE, FEP, PFA, ETFE, as well as specialty elastomers like FKM and FFKM—manufactured under strict pharmaceutical or medical device grade environments. These polymers excel in delivering low friction, chemical robustness, resistance to sterilization techniques, and long-term stability in body fluids.
Applications span from implantable device sliding surfaces and vascular access products, to pharmaceutical housings, high-purity filters, and diagnostic components. The market is served by global-scale chemical corporations, niche compounding specialists, and custom-molding partners. Distribution channels include direct OEM integration, regulatory-validated distributor networks, and value-added services like design for manufacturability and co-engineering. End-market dynamics are shaped by increasing surgical volumes, evolving device complexity, and stringent biocompatibility and quality standards.
Drivers
Key growth levers for the Medical Fluoropolymers Market include:
-
Technological Device Evolution: Devices with finer tolerances, tortuous paths, and precise manipulation (e.g., neurovascular microcatheters) demand coatings that reduce friction and withstand sterilization—capabilities well met by fluoropolymers.
-
Global Healthcare Infrastructure Build-Out: Rising medical facility investments in Asia, the Middle East, and Africa drive demand for medical-grade materials within equipment and disposables.
-
Stringent Patient Safety Norms: International guidelines for implant and device safety increasingly favor inert materials; fluoropolymers’ chemical stability aligns well with such demands.
-
Pharmaceutical Process Intensification: Strategies emphasizing continuous manufacturing and high-purity processing lines use fluoropolymer-based contact parts for cleanliness and resistance to aggressive chemicals.
-
Need for Durability Under Harsh Conditions: For devices exposed to high temperatures or sterilization cycles, fluoropolymers provide longevity unmatched by many conventional polymers.
Market Trends
-
Additive Manufacturing Integration: Experimental utilization of fluoropolymer powders in 3D printing enables rapid prototyping for custom medical components.
-
Bioactive Composite Surfaces: Combining fluoropolymers with active agents (e.g., anti‑microbial silver or antibiotic reservoirs) is enhancing implantation safety.
-
Lean, Solvent-Free Production: Manufacturers move to waterborne emulsions or solventless methods, reducing volatile organic compound emissions and improving sustainability profiles.
-
Smart Tracking & Serialization: Embedding QR codes or micro-tags in components improves traceability and recall control—critical in healthcare supply chains.
-
Flexible Compounding Solutions: Co‑formulations that blend fluoropolymers with elastomers or reinforcing fillers afford tunable mechanical behavior, enabling custom-performance parts.
Opportunities
Potential avenues for expansion in the Medical Fluoropolymers Market include:
-
Rapid Diagnostics and Wearables: Fluoropolymers with flexibility, sterilization compatibility, and barrier properties can support single-use biosensors and wearables for continuous monitoring.
-
Customized Microfluidic Cartridges: Short-run, tailor-made cartridges for point-of-care testing leverage fluoropolymer clarity and chemical resistance.
-
Collaborative R&D with Device Innovators: Co-creating novel catheter materials or sealants with medical device OEMs ensures first-mover access to innovative applications.
-
Environmental‑compliant Reformulation: Developing fluoropolymers that meet evolving environmental scrutiny—reduced PFAS content or recyclability—can appease ESG-conscious buyers.
-
Service & Support Bundling: Offering turnkey solutions (formulation, certification support, design assistance) increases customer value and loyalty.
Challenges
Despite promise, the Medical Fluoropolymers Market also faces several constraints:
-
Raw Material Volatility: Dependence on highly specialized monomers exposes companies to price swings and supply supply-chain shocks.
-
Evolving Environmental Legislation: Initiatives to regulate all PFAS compounds, even highly specialty ones, could result in increased compliance costs or restricted usage.
-
Regulatory Burden: Material validation for each new chemistry or grade requires extensive testing—costly and time-consuming for players seeking rapid innovation.
-
Competition from Alternative Polymers: Advanced non‑fluorinated low‑friction coatings (polyetheretherketone combinations, silicone‑based materials) offer potential substitutes in certain use‑cases.
-
Intellectual Property Barriers: Extensive patent protections in fluoropolymer chemistry may hinder new entrants or incremental innovation by smaller firms.
Recent Developments
Key recent activity in the Medical Fluoropolymers Market includes:
-
Expansion of medical-grade fluoropolymer production facilities in Asia-Pacific to better serve emerging market demand with localized supply.
-
Launch of new ultra-thin FEP coatings tailored for precision vascular interventions, offering lower friction coefficients and enhanced device navigability.
-
Introduction of waterborne high-purity PTFE dispersions that reduce emissions and accelerate component drying—improving production throughput and sustainability.
-
Strategic alliances formed between fluoropolymer scientists and medical device R&D centers to co-develop antimicrobial, drug-releasing catheter coatings.
-
Research announcements of new perfluoro‑elastomer formulations that withstand repeated autoclave cycles for use in implantable pumps and seals, extending device lifetimes.
Also Visit@ https://www.precedenceresearch.com
- Estrogen Replacement Therapy Market Size to Reach USD 19.46 Billion by 2034 - September 18, 2025
- Peptide Therapeutics CDMO Market Report Size, Share & Forecast 2034 - September 17, 2025
- Rare Musculoskeletal Disorder Treatments Market Size, Share & Future Trends 2034 - September 17, 2025
