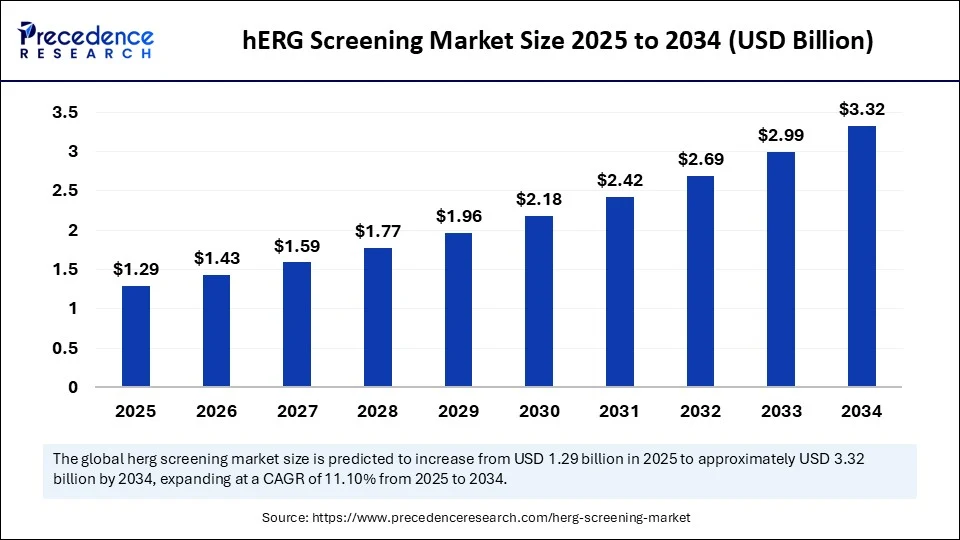
Market Overview
The hERG Screening Market is becoming a critical force in shaping the future of drug development. With cardiovascular safety at the core of regulatory approvals, hERG (human Ether-à-go-go-Related Gene) screening is no longer optional — it’s a cornerstone of preclinical evaluation. This gene codes for potassium ion channels in the heart, and its blockage by drug candidates can lead to fatal arrhythmias.
As pharmaceutical innovation accelerates, demand for efficient, accurate, and predictive toxicity screening is surging. The hERG Screening Market has evolved beyond traditional methods and is rapidly integrating new technologies to ensure faster, safer drug pipelines. From biotech startups to global pharmaceutical giants, stakeholders are investing heavily in cutting-edge hERG screening platforms.
The current momentum is fueled by a powerful combination of innovation, regulatory requirements, and increasing awareness about drug-induced cardiac risks. As a result, the hERG Screening Market is undergoing a major shift — from basic safety checks to an advanced, tech-powered industry segment critical to healthcare’s future.
AI and Innovation in the hERG Screening Market
Innovation is the engine driving the next chapter of the hERG Screening Market. Artificial Intelligence and machine learning are changing how cardiac toxicity is predicted. Algorithms trained on vast datasets can now model the likelihood of hERG inhibition with remarkable precision, drastically reducing reliance on expensive, slow lab-based processes.
AI isn’t just crunching numbers — it’s optimizing entire workflows. Predictive models can now flag risky compounds early in the development cycle, saving time and investment. Automated patch-clamp systems, paired with real-time analytics, offer deep insights into drug-ion channel interactions. These innovations are positioning the hERG Screening Market as a powerhouse of predictive toxicology.
Further advancements include the rise of robotic screening platforms and next-gen biosensor integration. These tools allow high-throughput screening with greater accuracy and less human error. As AI matures and becomes more accessible, it will continue to expand the capabilities of the hERG Screening Market, making cardiac safety assessment faster, smarter, and more affordable.
Future Trends in the hERG Screening Market
The hERG Screening Market is setting the pace for future-forward drug discovery. One of the biggest trends is the convergence of cardiotoxicity screening with organ-on-chip and multi-ion channel analysis. Rather than testing hERG in isolation, new platforms analyze multiple cardiac pathways, creating a more comprehensive safety profile.
Another trend is the growing use of hiPSC-derived cardiomyocytes. These stem cell-derived heart cells more accurately mimic human cardiac behavior, making them highly valuable for predictive modeling. This shift toward humanized models enhances the reliability of the hERG Screening Market.
Digital twin technologies — virtual replicas of biological systems — are starting to enter the space. These models simulate cardiac responses to drugs in silico, allowing companies to predict outcomes without animal testing. As digital tools, wearable tech, and computational biology evolve, they are set to push the hERG Screening Market into a new era of precision safety testing.
Get Sample Link@ https://www.precedenceresearch.com/sample/6531
Rising Demand in the hERG Screening Market
Demand for hERG screening is soaring, and it’s not hard to see why. The explosion of clinical trials, particularly in oncology, CNS, and cardiovascular segments, has intensified the need for early-stage safety profiling. As a result, the hERG Screening Market has become a go-to solution for pharma and biotech firms aiming to de-risk their pipelines.
Additionally, the increasing complexity of drug molecules, including biologics, peptides, and RNA-based therapies, has created new urgency for high-fidelity screening tools. Regulatory authorities are not only enforcing hERG testing but encouraging early incorporation into preclinical workflows.
The rise of CROs (Contract Research Organizations) and decentralized drug development models is also boosting demand. More companies are outsourcing hERG testing to specialized service providers, creating new segments within the hERG Screening Market. The net effect is a market on a steep growth trajectory, driven by necessity, innovation, and evolving healthcare demands.
Key Market Highlights
The hERG Screening Market is buzzing with activity. From technological breakthroughs to strategic mergers, the space is alive with momentum. Leading companies are launching new assay kits that combine faster readout times with improved sensitivity, reducing both cost and risk in drug development.
Strategic alliances are becoming increasingly common. Pharma companies are teaming up with tech firms to build proprietary hERG screening platforms. Cloud-based analysis tools and AI-powered dashboards are bringing real-time decision-making to the lab bench.
Another major highlight is the growing emphasis on automation. The transition from manual patch-clamp to fully automated systems is transforming efficiency in the hERG Screening Market, allowing researchers to process more compounds in less time without compromising quality.
Market Growth Drivers
The hERG Screening Market is riding a powerful wave of growth, and several forces are fueling this trajectory:
-
Stringent Regulatory Requirements: Global regulators mandate comprehensive hERG testing as part of drug approval, making it an essential service.
-
Expanding Drug Pipelines: More compounds mean more need for safety screening, directly benefiting the hERG Screening Market.
-
Cost-Saving Strategies: Early detection of cardiotoxicity avoids late-stage failures and saves millions in R&D expenses.
-
Technological Advancements: High-throughput systems, cloud analytics, and AI are making hERG screening faster and more accurate.
-
Growth of Biotech & CROs: Startups and research firms rely on outsourcing, expanding the service segment of the hERG Screening Market.
These factors are converging to create a market with immense momentum and future scalability.
Restraints
Despite its rise, the hERG Screening Market isn’t without challenges. One major restraint is the high capital investment required for advanced equipment. Automated patch-clamp systems and integrated platforms come with significant costs, which can be prohibitive for smaller players.
Another restraint is the complexity of data interpretation. Variability in assay responses and the nuances of cardiac physiology make it difficult to create standardized protocols. This can lead to inconsistencies in results across different labs, impacting regulatory trust in the data generated by the hERG Screening Market.
In addition, concerns around false positives — where non-toxic compounds are mistakenly flagged — can stall promising therapies and inflate development costs. Addressing these issues will be key to unlocking the full potential of the hERG Screening Market.
Opportunities
The future of the hERG Screening Market is bursting with opportunities. First, there’s immense untapped potential in emerging markets. As Asia-Pacific and Latin America expand their pharmaceutical manufacturing bases, the need for cardiac safety screening is growing quickly.
Next, there’s an opportunity in integrated safety testing. Companies offering bundled services that combine hERG screening with other ion channel, liver toxicity, and ADMET profiling will have a competitive edge.
Furthermore, the digital transformation of life sciences opens up possibilities for remote hERG data analysis, cloud storage, and AI-enabled screening-as-a-service platforms. These scalable solutions will empower even small biotech companies to access world-class screening capabilities, expanding the hERG Screening Market’s reach.
Finally, as personalized medicine grows, drug developers will need precision safety profiling tailored to specific patient populations. This trend will further drive innovation in the hERG Screening Market, making it a central component of future drug development strategies.
Regional Insights
-
North America remains the global leader in the hERG Screening Market, thanks to strong R&D infrastructure, high adoption of advanced screening tools, and strict FDA guidelines.
-
Europe is focused on ethical testing models and sustainable drug development. The use of humanized models like hiPSCs is growing rapidly, boosting innovation in the region’s hERG Screening Market.
-
Asia-Pacific is the fastest-growing region, driven by increased pharma outsourcing, government support for research, and the rise of biotech startups. Countries like China and India are investing heavily in lab automation and AI tools, making them key players in the future hERG Screening Market.
-
Latin America and the Middle East & Africa are showing potential due to improved healthcare systems and pharma investments. As regulatory frameworks strengthen, these regions are expected to play a more significant role in the global hERG Screening Market.
hERG Screening Market Companies
- Charles River Laboratories
- Eurofins Scientific
- Cyprotex (Evotec SE)
- Thermo Fisher Scientific
- Metrion Biosciences
- Aurora Biomed
- QPatch (Sophion Bioscience)
- Nanion Technologies
- Axol Bioscience
- B’SYS GmbH
- Multispan Inc.
- ChanTest (now part of Charles River)
- Bioneer Corporation
- Cellectricon
For inquiries about discounts, bulk purchases, or customization options, please reach out to us at sales@precedenceresearch.com
- Estrogen Replacement Therapy Market Size to Reach USD 19.46 Billion by 2034 - September 18, 2025
- Peptide Therapeutics CDMO Market Report Size, Share & Forecast 2034 - September 17, 2025
- Rare Musculoskeletal Disorder Treatments Market Size, Share & Future Trends 2034 - September 17, 2025
