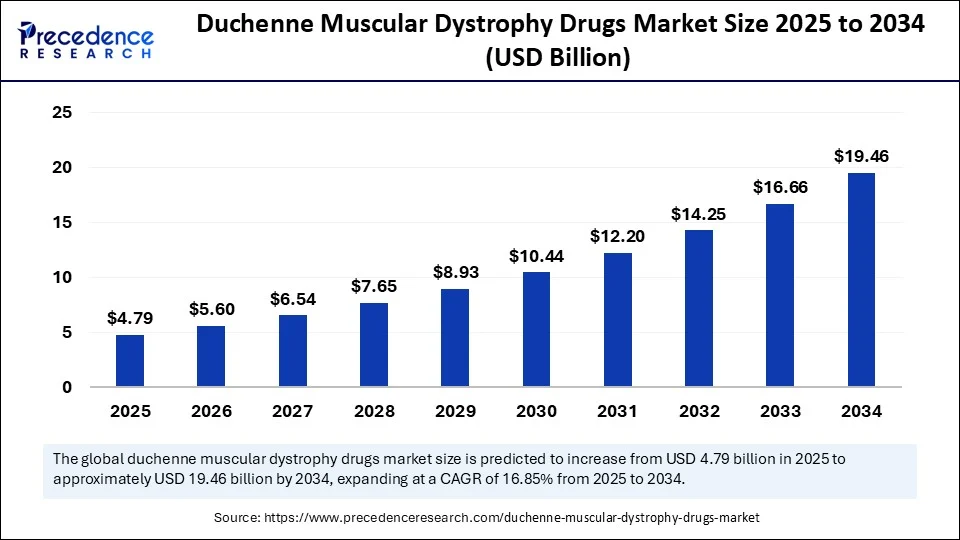
The global Duchenne muscular dystrophy drugs market was valued at approximately USD 4.1 billion in 2024 and is projected to reach nearly USD 19.5 billion by 2034, growing at a strong CAGR of 16.9% during the forecast period. This significant growth is attributed to continuous advancements in gene therapy, exon-skipping treatments, and corticosteroid-based therapies. Increasing research investments, regulatory incentives, and a growing understanding of genetic disorders are fueling innovation in this segment, transforming DMD from an underserved rare disease into an emerging field with high therapeutic potential.
Duchenne Muscular Dystrophy Drugs Market Key Insights
-
The global Duchenne muscular dystrophy (DMD) drugs market was valued at USD 4.1 billion in 2024 and is projected to reach USD 19.46 billion by 2034, growing at a CAGR of 16.85% from 2025 to 2034.
-
North America led the market with a dominant share of 46% in 2024, while the Asia Pacific region is anticipated to experience the fastest growth over the forecast period.
-
Based on treatment type, molecular-based therapies accounted for the largest market share at 43% in 2024.
-
The steroid-based therapies segment is projected to expand at a noteworthy CAGR of 16.25% through 2034.
-
In terms of distribution channel, hospital pharmacies held a substantial share of 42% in 2024.
-
The online pharmacies segment is expected to grow robustly, registering a CAGR of 16.74% during the forecast period.
Duchenne Muscular Dystrophy Drugs Market Growth Factors
Several factors are driving the market’s upward trajectory. Early diagnosis through expanded newborn screening programs is enabling timely intervention. Scientific advancements, especially in gene therapy and antisense oligonucleotides, are producing promising results in restoring partial dystrophin function. Regulatory support in the form of orphan drug designations, accelerated approvals, and tax benefits encourages pharmaceutical companies to invest in developing novel treatments. Moreover, strong patient advocacy and global awareness campaigns have contributed to improved patient engagement and clinical trial participation.
Key Market Drivers
The market is being propelled by breakthrough drug approvals and label expansions, which are increasing the eligible patient population for advanced therapies. For example, new approvals for gene therapy products targeting older or non-ambulatory patients have significantly broadened the market base. Additionally, the economic burden of DMD on healthcare systems is driving demand for treatments that can slow disease progression and reduce long-term care costs. Companies are also leveraging platform technologies that allow them to address multiple neuromuscular diseases, extending the commercial potential of their pipelines.
Opportunities
The future of the DMD drugs market holds numerous opportunities. Gene-editing tools like CRISPR and next-generation micro-dystrophin therapies offer the promise of potentially curative one-time treatments. Combination therapies that integrate corticosteroids with exon-skipping or other disease-modifying agents may improve patient outcomes and offer product lifecycle expansion. Furthermore, the integration of artificial intelligence in clinical trial design and patient stratification is expected to accelerate research and improve targeting, especially in genetically diverse populations.
Challenges
Despite the progress, the market faces considerable challenges. Safety concerns, especially those associated with high-dose gene therapies, have led to increased scrutiny and temporary suspensions of trials in the past. The high cost of therapies—some exceeding USD 3 million per dose—raises concerns about affordability, reimbursement, and equitable access. Moreover, the genetic diversity of DMD mutations means no single treatment fits all, complicating treatment strategies and increasing the need for precision medicine approaches. Manufacturing and scalability, particularly of viral vectors used in gene therapy, remain significant technical and economic hurdles.
Duchenne Muscular Dystrophy Drugs Market Regional Outlook
-
North America currently holds the largest market share, supported by strong regulatory frameworks, high healthcare expenditure, and rapid adoption of innovative treatments.
-
Europe follows, benefiting from centralized drug approval processes and public healthcare support, although pricing and reimbursement negotiations can delay access.
-
Asia-Pacific is expected to witness the fastest growth, driven by expanding genetic testing, increasing healthcare investments, and a rising number of clinical trials, particularly in countries like Japan, China, and South Korea.
-
Latin America and the Middle East & Africa currently represent smaller shares but are seeing gradual improvements in diagnosis rates and access to treatment through emerging public-private healthcare initiatives.
Duchenne Muscular Dystrophy Drugs Market Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 19.46 Billion |
| Market Size in 2025 | USD 4.79 Billion |
| Market Size in 2024 | USD 4.1 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 16.85% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Treatment, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Duchenne Muscular Dystrophy Drugs Market Segmental Insights
By Treatment:
-
Molecular-based therapies, including exon-skipping and mutation suppression drugs, dominated the market with a significant share in 2024. These precision treatments are gaining traction as they directly target the underlying genetic cause of DMD.
-
Corticosteroids, though traditional, remain a widely used treatment and are expected to continue growing due to their role in managing symptoms and delaying disease progression.
By Distribution Channel:
-
Hospital pharmacies accounted for the largest share in 2024 due to the need for physician supervision and clinical settings for administering gene and infusion-based therapies.
-
Online pharmacies are emerging rapidly, supported by digital health platforms and growing demand for convenient access to long-term medications like corticosteroids.
Duchenne Muscular Dystrophy Drugs Market Companies

- Aurobindo Pharma
- Capricor Therapeutics, Inc.
- Catalyst Pharmaceuticals, Inc.
- EspeRare Foundation
- FibroGen, Inc.
- ITALFARMACO S.p.A.
- NS Pharma, INC.
- PTC Therapeutics.
- Santhera Pharmaceuticals
- Sarepta Therapeutics, Inc.
Read Also: Veterinary Biomarkers Market
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344
- Arteriovenous Implants Market Enhance dialysis outcomes with advanced grafts, endovascular access, and AI-assisted precision - September 16, 2025
- Smart Retinal Implants Market Restore vision with wireless bioelectronic prosthetics and AI-powered retinal technologies - September 16, 2025
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025