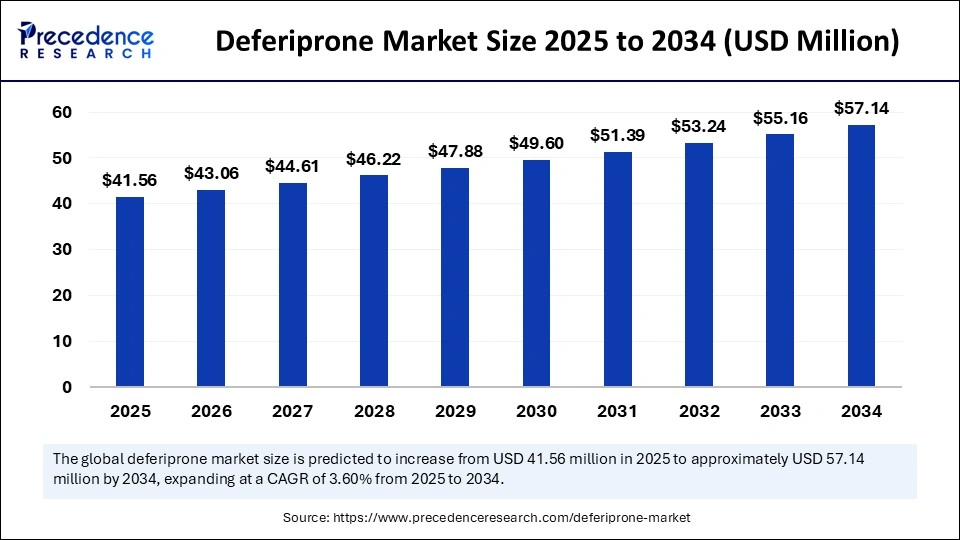
Deferiprone Market Key Points
-
In 2024, North America dominated the market, accounting for the largest share of 40%.
-
The Asia Pacific region is expected to grow at a notable pace throughout the forecast period.
-
Based on type, the tablets segment represented the highest share of 79% in 2024.
-
The oral solutions segment is projected to register the fastest growth rate between 2025 and 2034.
-
By therapeutic use, the iron overload disorders segment held the leading market share of 40% in 2024.
-
The thalassemia treatment segment is forecasted to grow at a considerable CAGR during the analysis period.
-
With respect to indication, the transfusional iron overload segment dominated the market with a 62% share in 2024.
-
The NTDT (non-transfusion-dependent thalassemia) caused overload segment is expected to experience rapid growth in the coming years.
Market Overview
The Deferiprone market is a crucial segment within the global pharmaceutical industry, focusing on the management of iron overload conditions, especially in patients who undergo regular blood transfusions. Deferiprone is an oral iron chelator approved primarily for the treatment of transfusional iron overload in patients with thalassemia major, sickle cell disease, and other chronic anemias. Its ability to bind excess iron and facilitate its excretion makes it a life-saving drug for individuals at risk of organ damage due to iron accumulation.
The market for Deferiprone has been gradually expanding due to the increasing prevalence of transfusion-dependent blood disorders globally. With growing awareness about iron toxicity and improved access to diagnostic facilities, more patients are being screened and treated for iron overload. Pharmaceutical companies are also investing in improving formulations, ensuring better patient compliance, and expanding indications beyond thalassemia, such as neurodegenerative diseases where iron dysregulation plays a role. The market is supported by both branded and generic versions, making it accessible across various socioeconomic groups and healthcare systems.
Growth Factors
Several key growth factors are driving the Deferiprone market. One of the primary factors is the rising incidence of genetic blood disorders, such as β-thalassemia and sickle cell anemia, especially in regions like Southeast Asia, the Middle East, and parts of Africa. These conditions often require chronic blood transfusions, which inevitably lead to iron accumulation. Deferiprone provides an essential therapeutic option for managing this burden, especially for patients who are unable to tolerate or respond adequately to other chelators like deferoxamine.
Another important growth factor is the shift toward oral medications in chronic disease management. Compared to parenteral chelators, Deferiprone offers the convenience of oral administration, leading to better adherence and outcomes, particularly in pediatric and adolescent populations. Additionally, research into Deferiprone’s potential neuroprotective applications in diseases like Parkinson’s and Friedreich’s ataxia, where iron accumulation in the brain is implicated, opens up new therapeutic areas and patient bases.
Regulatory approvals and orphan drug designations in major markets like the U.S. and Europe are also encouraging pharmaceutical companies to invest in Deferiprone and its expanded indications. Supportive healthcare policies and global health initiatives targeting rare diseases further strengthen market prospects.
Impact of AI on the Market
Artificial Intelligence (AI) is starting to reshape the Deferiprone market and the broader pharmaceutical space in transformative ways. AI is primarily being used to accelerate drug discovery and development, with machine learning algorithms analyzing molecular structures, predicting drug efficacy, and identifying off-label uses. This holds particular promise for Deferiprone, which may have broader applications in conditions involving abnormal iron metabolism or neurodegenerative disorders.
In clinical practice, AI-powered decision support tools are being deployed to optimize patient care. These systems can analyze patient histories, laboratory data, and genetic profiles to personalize treatment plans—deciding when and how to use Deferiprone, at what dosage, and in combination with which other therapies. This individualized approach improves efficacy and reduces the risk of adverse effects, such as agranulocytosis or gastrointestinal disturbances.
AI is also improving pharmacovigilance and drug safety monitoring, allowing real-time analysis of adverse event reports, electronic health records, and social media data to detect patterns and flag early safety signals. For a drug like Deferiprone, which has narrow safety margins in certain cases, this is especially valuable.
Furthermore, AI-driven platforms are being used to model disease progression and drug outcomes, enabling healthcare providers and researchers to better understand how iron chelation impacts long-term health, especially in complex, multi-organ conditions like thalassemia.
Market Drivers
The market is being driven by several powerful forces. Firstly, the growing burden of iron overload conditions, especially due to chronic transfusions in thalassemia and sickle cell patients, is a fundamental driver. As global diagnostic capabilities improve, more patients are being diagnosed and treated early, increasing the demand for effective chelation therapies like Deferiprone.
Improved awareness and screening programs in developing regions, often supported by governments or NGOs, are bringing more patients into the therapeutic fold. This is particularly impactful in regions with high rates of consanguinity and inherited blood disorders.
Additionally, expanding indications and off-label uses are increasing the drug’s relevance. For instance, studies are exploring the use of Deferiprone in treating iron accumulation in neurodegenerative diseases, opening new markets and patient segments.
Regulatory support and favorable reimbursement policies are also encouraging the adoption of Deferiprone, especially in high-income countries where orphan drug policies incentivize the development and marketing of rare disease treatments.
Opportunities
The Deferiprone market holds numerous promising opportunities. One of the most compelling is the exploration of neurological applications, where iron dysregulation is believed to contribute to diseases such as Parkinson’s, Alzheimer’s, and multiple sclerosis. Clinical research in this area could significantly expand the drug’s market potential.
Pediatric applications also present an opportunity. Deferiprone’s oral formulation makes it ideal for use in children, and further research into safety, dosing, and long-term outcomes in pediatric populations can drive greater adoption in this demographic.
Another opportunity lies in the emerging markets, where blood disorders are more prevalent, but access to care remains limited. Strategic partnerships with governments and health organizations can help improve drug access and affordability, significantly expanding the patient base.
Development of extended-release formulations or combination therapies could improve patient compliance and outcomes, differentiating products in an increasingly competitive market. Furthermore, as digital health and remote monitoring tools evolve, pharmaceutical companies can explore companion digital therapeutics to track iron levels and adherence.
Challenges
Despite its importance, the Deferiprone market faces several notable challenges. Safety concerns remain a critical issue, particularly the risk of neutropenia and agranulocytosis, which require frequent blood monitoring. This makes the drug less convenient than initially perceived and may deter adoption in areas with poor healthcare infrastructure.
Regulatory hurdles and pricing pressures also impact the market. As generic versions become more prevalent, especially in cost-sensitive regions, competition increases, placing pressure on profit margins. In high-income markets, payers are demanding real-world evidence and cost-effectiveness data to justify reimbursements.
Limited awareness and late diagnosis of iron overload conditions continue to hinder optimal market penetration in many developing regions. Without adequate screening and education programs, many eligible patients remain untreated or mismanaged.
Additionally, barriers in research and development for new indications, such as neurodegenerative diseases, include the complexity of clinical trials, lack of definitive biomarkers, and the slow pace of regulatory approval for repurposed drugs.
Regional Outlook
North America remains a key market for Deferiprone, supported by advanced healthcare infrastructure, strong regulatory frameworks, and higher awareness of thalassemia and related disorders. The U.S., in particular, has seen increased uptake following FDA approvals and the availability of both branded and generic versions.
Europe also represents a significant share, particularly in countries like Italy, Greece, and Cyprus, where thalassemia is relatively common. The region benefits from robust rare disease frameworks and collaborative research networks that support Deferiprone’s use and ongoing clinical evaluation.
Asia-Pacific is both the largest and fastest-growing region in terms of potential patient base, especially in countries like India, Thailand, Pakistan, and Indonesia, where inherited hemoglobin disorders are highly prevalent. Government initiatives and the entry of generics are making the drug more accessible, though infrastructure and affordability remain concerns.
The Middle East and North Africa (MENA) region shows significant potential due to high prevalence rates of thalassemia and consanguineous marriages. However, limited healthcare infrastructure and funding constraints pose barriers that need to be addressed through international aid and regional partnerships.
Latin America and Sub-Saharan Africa represent emerging opportunities, though market development in these regions will rely heavily on improvements in diagnostics, health policy support, and international collaboration to improve access.
Deferiprone Market Companies

- Apotex Inc.
- Chiesi Farmaceutici S.p.A.
- Cipla Limited
- Novartis International AG
- Sun Pharmaceutical Industries Ltd.
- Taro Pharmaceutical Industries Ltd.
- VHB Life Sciences Limited
- Zydus Cadila
Segments Covered in the Report
By Type
- Capsule
- Oral Solutions
- Tablets
By Therapeutic Use
- Iron Overload Disorders
- Sickle Cell Disease Treatment
- Thalassemia Treatment
By Indication
- NTDT Caused Overload
- Transfusional Iron Overload
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Read Also: PEEK Interbody Devices Market
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6097
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344
- Arteriovenous Implants Market Enhance dialysis outcomes with advanced grafts, endovascular access, and AI-assisted precision - September 16, 2025
- Smart Retinal Implants Market Restore vision with wireless bioelectronic prosthetics and AI-powered retinal technologies - September 16, 2025
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025
