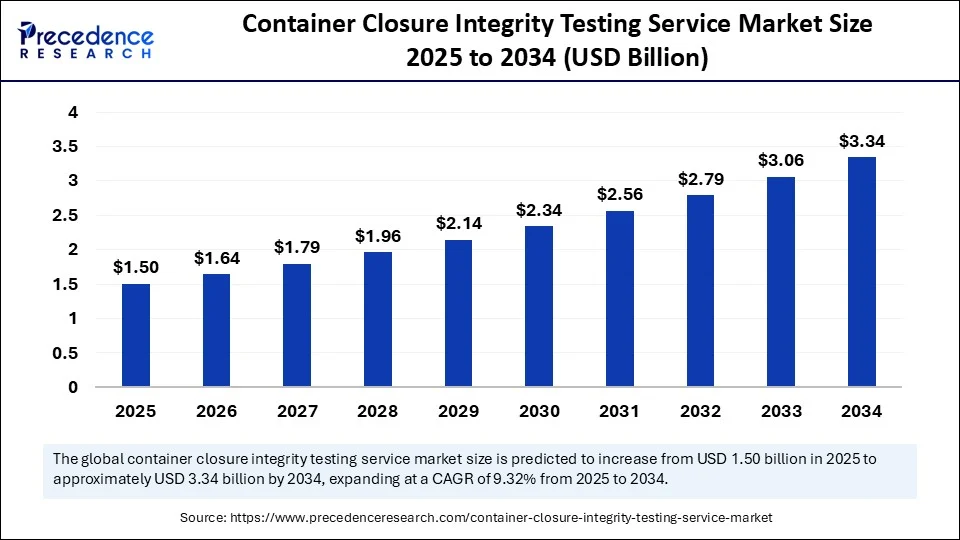
Market Overview
The Container Closure Integrity Testing Service Market has emerged as a critical support industry for pharmaceutical, biotech, and medical device manufacturers. With increasing scrutiny from global regulatory bodies, the demand for airtight, sterile, and leak-free containers has never been greater.
As pharmaceutical companies expand globally and release complex biologics and injectables, the Container Closure Integrity Testing Service Market plays a crucial role in ensuring packaging quality, patient safety, and compliance with current good manufacturing practices (cGMP). Whether during initial drug development or commercial production, these services are integral to the entire pharmaceutical lifecycle.
AI and Innovation in the Market
Technology is reshaping the Container Closure Integrity Testing Service Market with cutting-edge advancements in automation and artificial intelligence. AI-powered leak detection systems can now identify even microscopic defects in packaging, significantly reducing the margin of error and eliminating manual inconsistencies.
Automated image recognition, paired with high-resolution cameras and deep learning algorithms, improves test accuracy and speeds up batch inspections. As the market becomes increasingly digitized, companies are deploying robotic arms, real-time analytics, and data-driven quality monitoring tools to streamline the container closure integrity testing process.
Furthermore, AI allows predictive maintenance of testing systems, optimizing uptime and reducing operational disruptions, giving the Container Closure Integrity Testing Service Market a competitive, tech-driven edge.
Future Trends of the Market
The Container Closure Integrity Testing Service Market is expected to evolve rapidly in the coming years. A few key trends that will define its future include:
-
Shift toward non-destructive methods: Techniques like helium mass spectrometry and laser-based headspace analysis are replacing traditional destructive testing, preserving valuable drug products.
-
Smart data integration: As quality teams demand more traceability and auditability, service providers will increasingly use centralized data platforms for test validation and storage.
-
Remote testing services: With the rise of decentralized manufacturing, remote and mobile CCIT services will become more prevalent, especially in emerging markets.
-
Growth in biologics and combination products: These drug formats will fuel demand for custom CCIT services that can handle unique packaging formats and sensitivities.
Rising Demands of the Market
One of the primary drivers of the Container Closure Integrity Testing Service Market is the exploding demand for sterile injectable products. Injectable drug formulations are vulnerable to contamination, making closure integrity a non-negotiable requirement.
Additionally, the global COVID-19 pandemic has heightened the demand for vaccines and biologics, where packaging reliability directly impacts product efficacy. This has brought increased attention to the Container Closure Integrity Testing Service Market, particularly in regard to cold chain storage and transport.
Moreover, the growth of outsourcing in pharmaceutical manufacturing has pushed demand toward third-party CCIT providers. As more companies shift focus toward core R&D and commercialization, they rely on external partners to handle testing with regulatory-grade precision.
Key Market Highlights
Key developments that define the Container Closure Integrity Testing Service Market include:
-
Strategic expansions by leading service providers to enter new markets and regions.
-
Mergers and acquisitions among CDMOs and CCIT labs, enabling integrated service delivery.
-
New product launches in high-throughput and automated testing systems.
-
Increased demand for cleanroom-integrated services, ensuring on-site, sterile testing conditions.
These developments are shaping the dynamics of the Container Closure Integrity Testing Service Market, positioning it as a critical player in global pharmaceutical operations.
Market Growth Drivers
Several powerful forces are propelling the growth of the Container Closure Integrity Testing Service Market, including:
-
Rising regulatory pressure: Agencies like the FDA, EMA, and WHO are tightening standards around container closure testing, especially for parenteral drugs.
-
Complex drug formulations: Biologics, vaccines, and combination therapies require specialized packaging, increasing the need for customized testing protocols.
-
Outsourcing trends: Pharma companies are relying on contract service providers for faster market delivery and to reduce in-house QC burdens.
-
Increased global drug production: With pharmaceutical capacity increasing worldwide, especially in Asia-Pacific, the demand for container testing services continues to climb.
-
Focus on patient safety: Regulatory agencies and manufacturers alike are committed to preventing contamination-related recalls, boosting long-term demand for CCIT services.
These growth drivers ensure that the Container Closure Integrity Testing Service Market remains integral to pharmaceutical supply chains and regulatory compliance.
Also Read@ https://www.pharma-geek.com/dehydration-monitoring-devices-market/
Restraints
-
High cost of testing infrastructure: Investment in advanced equipment and facilities can be prohibitive for smaller labs and companies.
-
Technical complexity: Some drug formats, such as lyophilized products or dual-chamber syringes, present unique challenges for standard testing procedures.
-
Regulatory complexity: Variations in global compliance requirements add complexity to international testing operations.
-
Lack of skilled labor: Operating sophisticated CCIT equipment and interpreting results requires trained personnel, which are in short supply in many regions.
-
Slow adoption of newer technologies: In conservative markets, legacy testing methods are still preferred over modern techniques, slowing innovation adoption.
These restraints may delay the full potential of the Container Closure Integrity Testing Service Market, especially in underdeveloped regions.
Opportunities
-
Emerging market growth: Countries with growing pharmaceutical production—such as India, Brazil, and Indonesia—present ripe opportunities for CCIT expansion.
-
Tailored testing for biologics: As biologics become more mainstream, there’s a growing need for testing services specifically designed for sensitive containers.
-
Cloud-based lab data management: Leveraging cloud technology can streamline compliance documentation, improve audit trails, and enable remote oversight.
-
Environmental packaging: The rise of sustainable packaging materials opens a new frontier in CCIT, requiring adapted test methods.
-
On-demand CCIT services: Flexible, rapid-response CCIT offerings are expected to grow, especially for startups and short-run production lines.
Regional Insights
North America
The North American region leads the Container Closure Integrity Testing Service Market, driven by a mature pharmaceutical landscape and early adoption of technology. U.S.-based firms have strong ties to regulatory agencies and often act as benchmarks for global CCIT practices.
Europe
Europe remains a significant player, with countries like Germany, Switzerland, and the UK leading CCIT innovation. Stringent EMA regulations and a growing biosimilar market ensure continued demand.
Asia-Pacific
Asia-Pacific is experiencing a surge in demand due to increased pharmaceutical exports, favorable government policies, and rising investment in local drug manufacturing. India and China are key contributors to this growth.
Latin America & Middle East
While smaller, these regions show increasing CCIT service demand. Improvements in healthcare infrastructure and local manufacturing are supporting gradual market development.
Container Closure Integrity Testing Service Market Companies

- SGS SA
- Nelson Labs
- Eurofins Scientific
- West Pharmaceutical Services
- Lighthouse Instruments
- PTI (Packaging Technologies & Inspection)
- Pfeiffer Vacuum
- Stevanato Group
- Qualitest
- WIL Research Laboratories
- Mocon, Inc.
- Albany Molecular Research Inc. (AMRI)
- Labcorp Drug Development
- Cormica
- Nelson Analytical
- Gateway Analytical
- Toxikon Corporation
- Estrogen Replacement Therapy Market Size to Reach USD 19.46 Billion by 2034 - September 18, 2025
- Peptide Therapeutics CDMO Market Report Size, Share & Forecast 2034 - September 17, 2025
- Rare Musculoskeletal Disorder Treatments Market Size, Share & Future Trends 2034 - September 17, 2025
