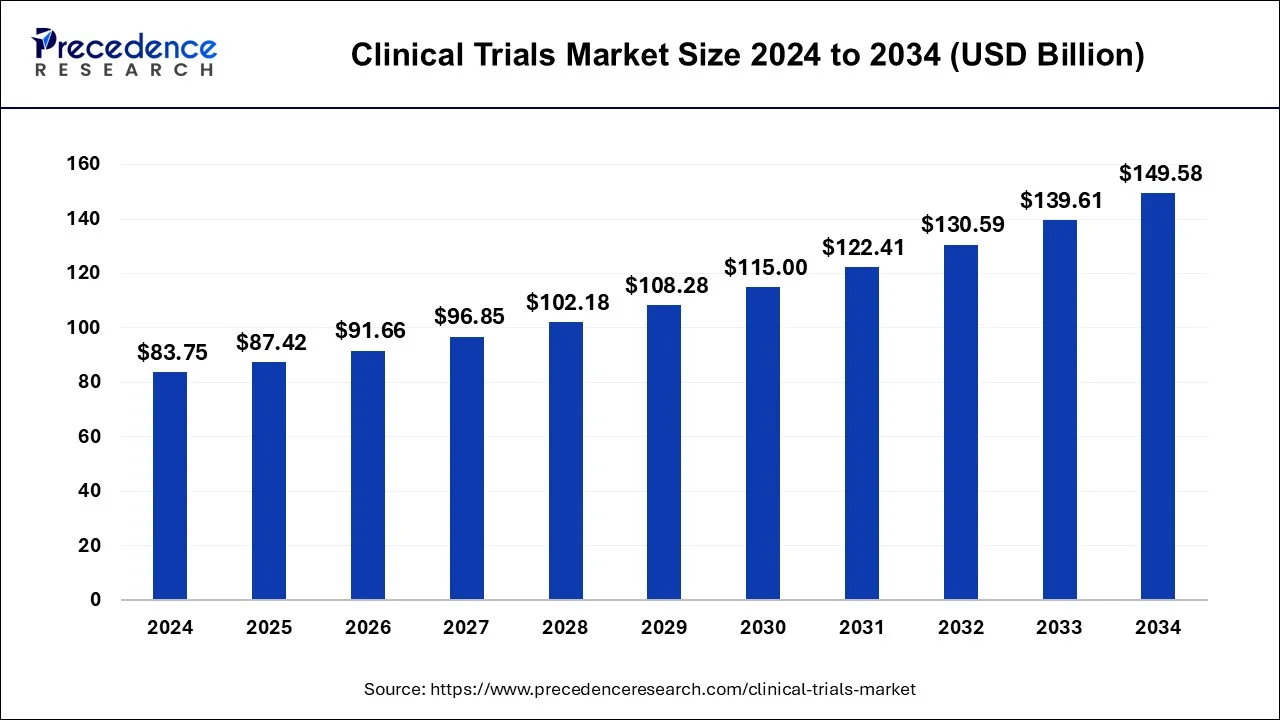
The global clinical trials market was valued at USD 83.75 billion in 2024 and is projected to reach USD 149.58 billion by 2034, growing at a CAGR of 6.10% from 2025 to 2034. The market expansion is driven by the rising prevalence of chronic diseases, adoption of advanced treatments like personalized medicine, and increasing outsourcing to Contract Research Organizations (CROs).
Read Also: U.S. Liquid Biopsy Market
Market Overview
Clinical trials are a critical step in clinical research, following strict protocols to assess the safety and efficacy of drugs, therapies, or interventions. Trials are conducted in multiple phases and provide detailed plans for procedures, tests, medications, and study durations.
Increasing drug development costs and the need for efficient trial management have encouraged pharmaceutical and biotech companies to outsource clinical research to CROs, which now offer services from study design to bio-statistical analysis and clinical trial management.
Market Drivers
-
Adoption of New Technology:
-
Digitization, e-COA systems, and remote monitoring streamline clinical trials and reduce errors, increasing efficiency and success rates.
-
-
Shift Towards Personalized Medicine:
-
Focus on patient-specific drug effects rather than the traditional “one size fits all” approach.
-
Growth in pharmacogenetics enhances the drug approval pipeline.
-
-
Rising Disease Variation and Prevalence:
-
Diverse disease profiles, especially in emerging countries, facilitate patient recruitment and the study of rare/orphan diseases.
-
-
Increasing Collaboration in Biomedical Research:
-
Biopharmaceutical companies collaborate to share costs and risks, particularly in areas like immuno-oncology.
-
U.S. Clinical Trials Market
The U.S. clinical trials market was valued at USD 45.07 billion in 2024 and is projected to reach USD 82.79 billion by 2034, at a CAGR of 6.40%. North America dominates globally due to its advanced healthcare infrastructure, strong regulatory frameworks, and high R&D spending.
Regional Insights
-
North America: Largest market – high incidence of chronic diseases and advanced infrastructure.
-
Europe: USD 18.74 billion in 2024; CAGR 5.5%
-
Asia Pacific: Fastest-growing – USD 11.86 billion in 2024; CAGR 6.7%
-
Driven by cost advantages, rapid patient recruitment, regulatory updates, and digital trial adoption.
-
-
MEA: USD 859.6 million in 2024; CAGR 4.1%
China leads APAC with 25,000+ trials in 2024, benefiting from regulatory changes and government and private sector investments.
Driving Factors for Clinical Trials Market Growth
-
Adoption of New Technology in Clinical Research
-
Digitization and advanced software (e.g., electronic clinical outcome assessment, AI-driven monitoring) streamline trial processes.
-
Enhances accuracy, reduces errors, and speeds up data collection, making trials more efficient.
-
Promotes faster regulatory compliance and reduces operational costs for sponsors.
-
-
Shift Towards Personalized Medicine
-
Traditional trials treat all patients uniformly, but personalized medicine targets individual responses to therapies.
-
Use of pharmacogenetics helps identify which drugs work best for specific patients, improving trial success rates.
-
Encourages investment in patient-centric trials, increasing the clinical trial pipeline.
-
-
Growing Disease Variation and Prevalence
-
Rising global incidence of chronic and rare diseases drives the need for new treatments and therapies.
-
Diverse patient populations enable faster recruitment and better representation of disease demographics.
-
“Orphan disease” incentives in markets like the U.S. promote trials for rare diseases.
-
-
Increasing Collaboration in Biomedical Research
-
Biopharma companies form alliances and partnerships to share resources and reduce risks of costly clinical trials.
-
Collaborative trials, including combination therapies like immuno-oncology, improve efficiency and innovation.
-
-
Outsourcing to Contract Research Organizations (CROs)
-
Rising R&D costs and regulatory complexities push pharmaceutical companies to outsource trial services.
-
CROs offer end-to-end services including study design, site management, data analysis, and patient recruitment.
-
This expands access to global patient populations and accelerates trial timelines.
-
Market Segmentation
By Study Design
-
Interventional: Largest share – assessing efficacy and safety of new therapies.
-
Expanded Access: Fastest-growing – providing emergency access to investigational treatments.
By Indication
-
Oncology: Largest share – focus on cancer treatments and therapies.
-
CNS Conditions: Fastest-growing – covers neurological disorders like Parkinson’s, epilepsy, and ALS.
By Service Type
-
Laboratory Services: Largest segment – crucial for drug development and testing.
-
Decentralized Clinical Trial Services: Fastest-growing – facilitates remote trial management, real-world data collection, and faster recruitment.
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
- Protein Expression Market Size To Reach Around USD 12.27 Bn By 2034 - October 8, 2025
- Clinical Trials Market Size to Reach USD 149.58 Billion by 2034 - October 8, 2025
- U.S. Liquid Biopsy Market Size To Hit USD 6.62 Billion By 2034 - October 8, 2025
