Clinical Trials Market Set for Steady Growth Driven by Innovation and Global Expansion
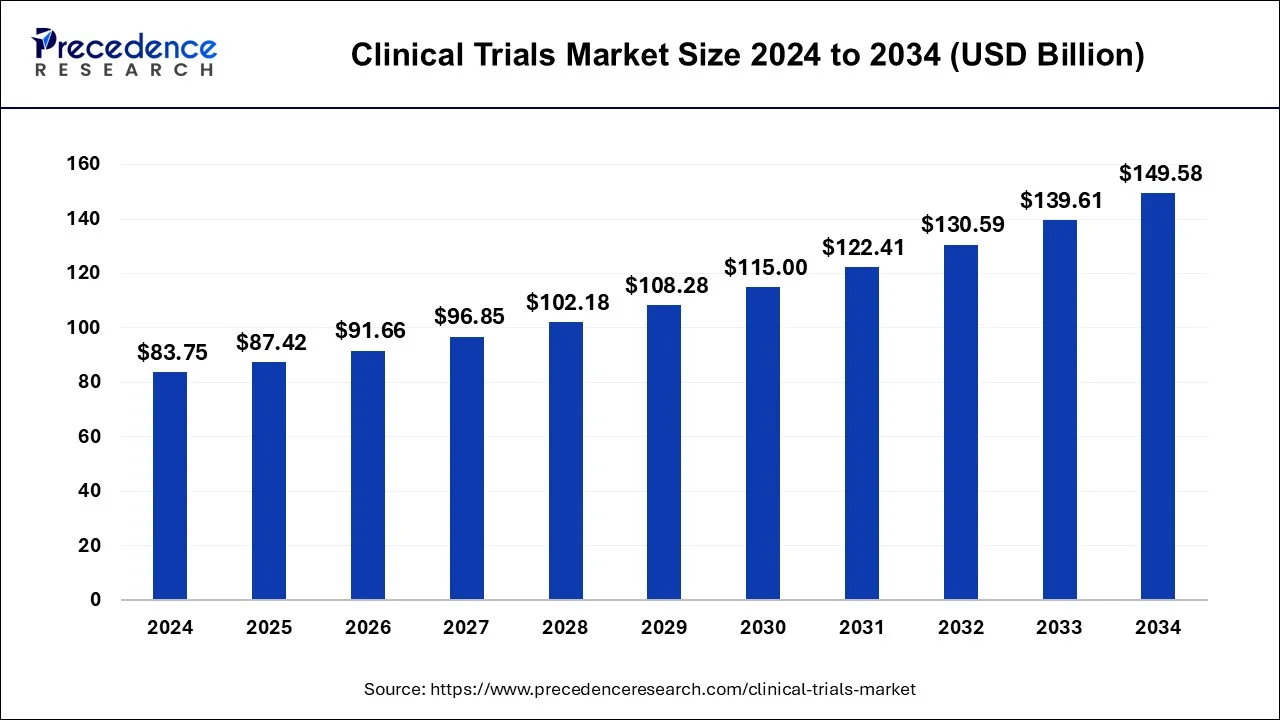
The global clinical trials market is undergoing a transformative shift driven by cutting-edge technologies, growing healthcare demands, and the rising complexity of drug development. Estimated at USD 83.75 billion in 2024, the market is projected to grow at a CAGR of 6.10%, reaching approximately USD 149.58 billion by 2034. This impressive growth is fueled by several key trends—personalized medicine, digital trials, increasing outsourcing, and the global expansion of research sites.
Driving Forces Behind Market Expansion
One of the most powerful accelerators of market growth is the increasing prevalence of chronic and rare diseases, prompting a surge in clinical trial activity worldwide. At the same time, pharmaceutical and biotech companies are facing growing pressure to bring safe and effective drugs to market faster and more cost-effectively. This pressure has led to a paradigm shift in trial models, marked by
-
Digitization of trial processes, including the use of electronic clinical outcome assessments (e-COA) and AI-driven analytics
-
Decentralized clinical trials (DCTs) that enable remote participation and monitoring
-
Outsourcing to CROs (Contract Research Organizations) for design, management, analytics, and regulatory support
Regional Insights: North America Leads, Asia Pacific Surges
North America continues to dominate the market with a 59.31% share in 2024, thanks to its robust healthcare infrastructure, favorable regulatory landscape, and high R&D investment. The U.S. alone hosts more than 130,000 trials, supported by a strong network of NIH-funded institutions and regulatory oversight from the FDA.
However, Asia Pacific is emerging as the fastest-growing region, with a projected CAGR of 6.7%. Countries like China and India are becoming global hotspots due to their cost efficiency, rapid patient recruitment, and increasingly favorable regulatory frameworks. In fact, 25% of all new global trials are now initiated in Asia Pacific.
The Rise of Personalized Medicine and Collaborative Research
The shift towards personalized medicine—tailoring treatments based on individual genetic profiles is reshaping clinical research. Unlike traditional trials conducted on thousands of patients, personalized medicine requires highly targeted trials supported by pharmacogenetics. This change is increasing the number of pipeline drugs reaching market, while reducing the failure rate in trial phases.
Moreover, collaborative models are becoming increasingly common. Pharmaceutical companies are partnering with peers and CROs to share resources and mitigate the high costs of drug development. Notable partnerships, like those in immuno-oncology between MSD and Lilly, reflect the growing importance of synergy in tackling complex diseases.
Final Thoughts
The clinical trials market is at a pivotal point in its evolution. As the healthcare landscape becomes more complex and interconnected, the demand for faster, more adaptive, and patient-centric trials will only increase. Digitization, globalization, and a shift toward personalized treatment are not just trends—they are becoming the new standard.
At Precedence Research, we continuously monitor these developments to provide reliable, data-driven insights that empower organizations to make informed decisions in this dynamic industry.
Clinical Trials Market Key Players
- Parexel
- IQVIA
- Charles River Laboratory
- Omnicare
- Kendle
- Chiltern
- Pharmaceutical Product Development, LLC
Segments Covered in the Report
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Autoimmune/Inflammation
- Rheumatoid arthritis
- Multiple Sclerosis
- Osteoarthritis
- Irritable Bowel Syndrome (IBS)
- Others
- Pain Management
- Chronic Pain
- Acute Pain
- Oncology
- Blood Cancer
- Solid Tumors
- Other
- CNS Condition
- Epilepsy
- Parkinson’s Disease (PD)
- Huntington’s Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
- Diabetes
- Obesity
- Cardiovascular
- Others
By Service Type
- Laboratory Services
- Bioanalytical Testing Services
- Decentralized Clinical Trial Services
- Patient Recruitment
- Site Identification
- Analytical Testing Services
- Clinical Trial Supply & Logistic Services
- Other Services
By Geography
- North America
- Europe
- Asia-pacific
- Latin America
- Middle and East Africa
To access the full market report, company profiles, and in-depth trends analysis, visit sales@precedenceresearch.com
- Autonomous Vehicle Market Size to Reach USD 4450.34 Billion by 2034 - September 19, 2025
- Sterilization Equipment Market Size to Reach USD 36.16 Bn by 2034 - September 19, 2025
- Wearable Cardiac Devices Market Size to Hit USD 32.16 Bn by 2034 - September 19, 2025