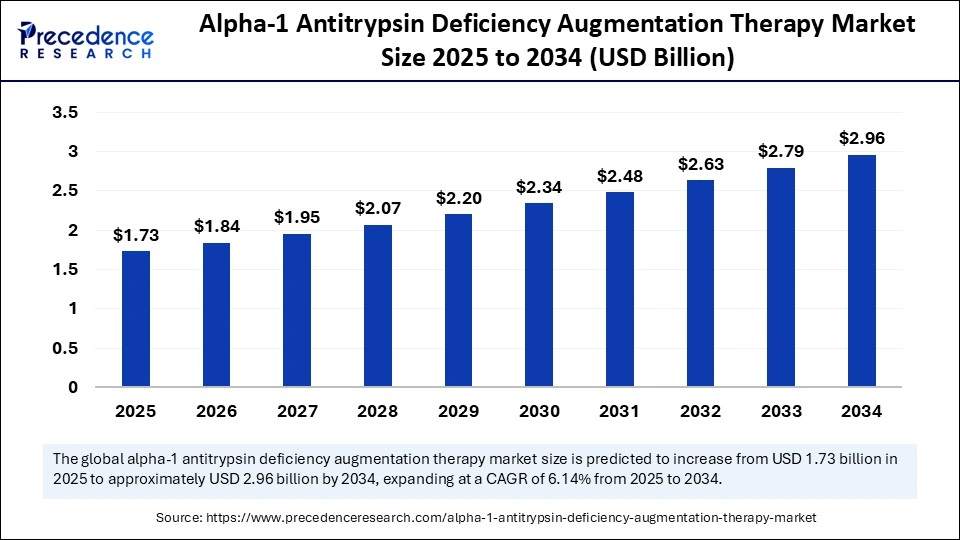
Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market Key Highlights
-
North America led the global market with the highest share of 42% in 2024.
-
Asia Pacific is projected to grow at the fastest CAGR between 2025 and 2034.
-
By product type, the Prolastin C segment accounted for the largest market share in 2024.
-
The Zemaira/Respreeza segment, by product type, is expected to grow at a significant CAGR in the coming years.
-
By end-user, the hospitals segment held the largest market share in 2024.
-
The specialty clinics segment, by end-user, is anticipated to expand at a notable CAGR over the projected period.
Clinical Relevance & Drivers
Alpha‑1 antitrypsin deficiency is a genetic condition that results in low levels of the AAT protein, which is responsible for protecting the lungs from neutrophil elastase, an enzyme that can cause tissue damage. Individuals with this deficiency, especially those with the severe PiZZ genotype, are at heightened risk for early-onset emphysema, chronic obstructive pulmonary disease (COPD), and liver disease. Augmentation therapy involves the intravenous administration of purified AAT protein derived from human plasma, helping to restore normal protein levels and protect the lungs.
The market is driven by the increasing prevalence of COPD, a broader recognition of rare diseases in healthcare systems, advancements in plasma fractionation technologies, and favorable regulatory support for orphan drugs. Increased clinician awareness and growing emphasis on genetic screening programs have also supported early diagnosis and treatment, further accelerating demand for augmentation therapy.
Treatment Modalities & Innovation
Currently, augmentation therapy is the only approved pharmacological treatment specifically designed for AAT deficiency-related lung disease. Key products on the market include Prolastin C, Glassia, Aralast NP, Zemaira, and Respreeza, each offering slightly different dosing schedules and formulation profiles. These therapies have demonstrated efficacy in slowing the progression of emphysema in patients with severe AAT deficiency. Innovation within this space is focused on developing recombinant or synthetic AAT alternatives that can reduce the dependency on human plasma-derived products.
Additionally, research is ongoing into gene therapy solutions that could offer a long-term or potentially curative approach. Digital health solutions and telemedicine integration are also enhancing therapy management, enabling real-time monitoring, virtual follow-ups, and personalized dosing to improve adherence and outcomes.
Alpha 1 Antitrypsin Deficiency Augmentation Therapy Market Scope & Segmentation
The Alpha‑1 antitrypsin deficiency augmentation therapy market serves patients across a broad geographic scope, including North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America leads the market, largely due to advanced healthcare infrastructure, active patient registries, and reimbursement frameworks that support long-term therapy. Europe follows closely, with established clinical guidelines and consistent product availability.
Asia Pacific is emerging as a high-potential region, propelled by growing awareness, increased screening initiatives, and expanding access to biologic treatments. The market is segmented by route of administration (primarily intravenous), distribution channels (hospital pharmacies, specialty clinics, and home care), and end users (hospitals, outpatient clinics, and individuals via home infusion services). This segmentation ensures a tailored approach to therapy delivery based on regional healthcare dynamics.
Alpha 1 Antitrypsin Deficiency Augmentation Therapy Market Competitive & Cost Dynamics
- Takeda Pharmaceutical Company Limited
- CSL Behring
- Paramount Therapeutics
- Vertex Pharmaceuticals Incorporated
- Shire plc
- Chiesi Farmaceutici SpA
- Shanghai RAAS Biotechnology Co.
- JCR Pharmaceuticals Co.
- Instituto Butantan
- Lonza Group Ltd
- Samsung BioLogics
- Boehringer Ingelheim
As a result, pricing and access remain central issues in many markets. Companies are working to address these concerns through cost-sharing models, expanded manufacturing capacities, and the exploration of biosimilar or recombinant formulations. In parallel, smaller biopharmaceutical firms are entering the field with novel delivery mechanisms, including inhaled therapies and long-acting injectables, aimed at enhancing convenience and broadening patient access.
Future Outlook & Challenges
The future of the Alpha‑1 antitrypsin deficiency augmentation therapy market is promising, but not without its challenges. Diagnostic delays and underdiagnosis continue to limit patient access to therapy, particularly in countries without active screening programs. Additionally, the dependence on plasma-derived proteins makes supply chains vulnerable to fluctuations in donor availability. Overcoming these hurdles will require coordinated efforts in awareness, education, and investment in synthetic biologics or gene-based alternatives.
Nonetheless, the emergence of digital health tools, wearable devices for real-time symptom tracking, and AI-powered diagnostics will likely contribute to earlier detection and improved disease management. Continued investment in clinical trials, coupled with a supportive regulatory environment for orphan drugs, will drive therapeutic innovation and expand the market landscape over the next decade.
Segments Covered in the Report
By Product Type
- Glassia
- Aralast NP
- Prolastin C
- Zemaira/Respreeza
- Others
By End-User
- Hospitals
- Speciality Clinics
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Read Also: Nebivolol Tablets Market
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6241
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344
- Arteriovenous Implants Market Enhance dialysis outcomes with advanced grafts, endovascular access, and AI-assisted precision - September 16, 2025
- Smart Retinal Implants Market Restore vision with wireless bioelectronic prosthetics and AI-powered retinal technologies - September 16, 2025
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025
