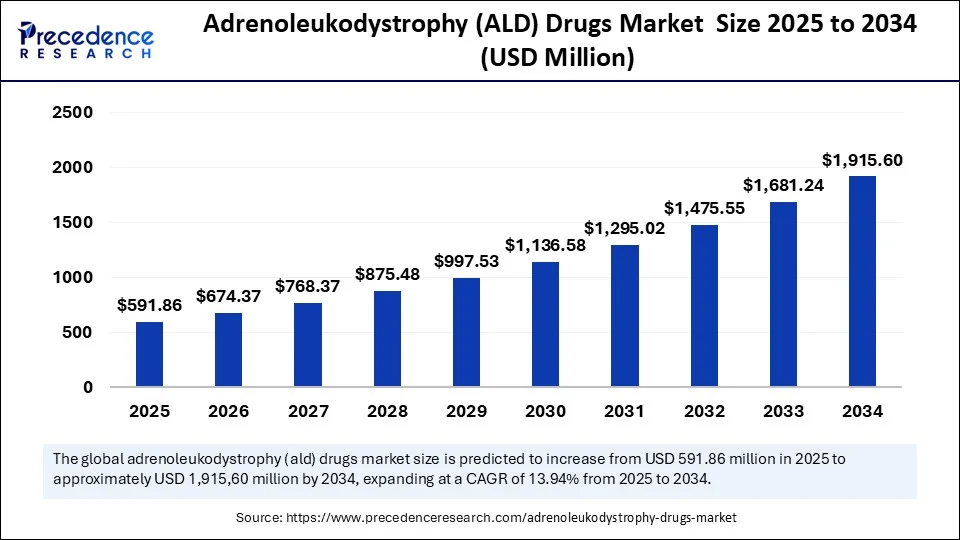
The global adrenoleukodystrophy drugs market size was evaluated at USD 519.45 million in 2024 and is predicted to climb around USD 1,915.60 billion by 2034, at a CAGR of 13.94%.
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6351
Adrenoleukodystrophy (ALD) Drugs Market Key Highlights
-
The global adrenoleukodystrophy (ALD) drugs market was valued at USD 519.45 million in 2024 and is projected to reach USD 1,915.60 million by 2034, growing at a CAGR of 13.94% from 2025 to 2034.
-
North America led the market in 2024, accounting for 44% of the global share.
-
The Asia-Pacific region is expected to witness the fastest growth during the forecast period.
-
By treatment type, the gene therapy (lenti-based) segment held the largest share at 38% in 2024, while the AAV-based gene therapy segment is poised to grow at a significant CAGR.
-
Based on disease type, childhood cerebral ALD dominated the market with a 52% share in 2024, whereas the adrenomyeloneuropathy (AMN) segment is projected to grow at the highest CAGR.
-
In terms of route of administration, the intravenous (IV) segment accounted for the largest market share of 47% in 2024, while the intrathecal segment is anticipated to grow at the fastest rate through 2034.
-
By distribution channel, hospital pharmacies held a significant share of 40% in 2024, and the online pharmacies segment is expected to register the fastest CAGR in the coming years.
Adrenoleukodystrophy (ALD) Drugs Market Overview
The adrenoleukodystrophy drugs market, though relatively small compared with mainstream neurological segments, has gained strategic importance as rare‑disease therapeutics attract greater scientific and commercial attention.
This acceleration is being propelled by regulatory incentives for orphan drugs, heightened awareness among clinicians and patient‑advocacy groups, and a pipeline rich in gene‑replacement vectors, cell therapies, and next‑generation lipid‑modulating agents. ALD—an X‑linked peroxisomal disorder characterized by the toxic buildup of very‑long‑chain fatty acids (VLCFAs) in adrenal and central nervous system tissues—has historically been managed only with supportive endocrine care and the limited efficacy of Lorenzo’s oil.
Today, however, disease‑modifying therapies aimed at restoring ABCD1 transporter function or directly correcting the underlying genetic defect are poised to reshape the treatment landscape, driving significant value creation in this niche market.
Adrenoleukodystrophy Drugs Market Growth Factors
Several structural trends underpin the market’s robust growth trajectory. First, expanded newborn‑screening programs in North America and parts of Europe now routinely include ALD, enabling presymptomatic detection and earlier intervention—broadening the treatable population and extending therapeutic windows.
Second, advances in lentiviral and AAV‑mediated gene therapy have reached late‑stage clinical trials, signaling an impending shift from symptomatic management to potential one‑time curative treatments. Third, specialty pharmaceutical companies are increasingly leveraging priority‑review vouchers, orphan exclusivity, and fast‑track designations to accelerate development timelines and secure premium pricing.
Finally, increased venture capital and strategic partnering among biotech firms have infused fresh funding into ALD research, encouraging a wave of small‑molecule explorations targeting VLCFA synthesis and degradation pathways.
Role of AI in the Adrenoleukodystrophy Drugs Market
Artificial intelligence is emerging as a catalyst at multiple stages of the ALD drug‑development lifecycle. In early discovery, AI‑driven molecular modeling accelerates the identification of compounds that modulate fatty‑acid elongase enzymes or boost peroxisomal β‑oxidation. Predictive analytics aid in selecting optimal viral vectors and promoter sequences for gene therapy, shortening iteration cycles and enhancing safety profiles. In clinical development, machine‑learning algorithms analyze multimodal patient data—MRI lesion scores, plasma VLCFA levels, and genetic modifiers—to stratify trial participants and predict therapeutic response, thereby reducing trial sizes and duration.
Post‑approval, AI‑enabled real‑world evidence platforms can monitor long‑term neurological outcomes, adrenal function, and safety signals, providing regulators and payers with robust evidence of value and facilitating outcomes‑based reimbursement models.
Adrenoleukodystrophy Drugs Market Regional Outlook
North America currently accounts for the largest revenue share, underpinned by widespread newborn screening, concentrated centers of excellence, and payer systems more amenable to high‑cost orphan therapies.
Europe follows, buoyed by strong rare‑disease policies and cross‑border pediatric neurology networks, though heterogeneous reimbursement negotiation across member states can slow rollout.
The Asia‑Pacific region is anticipated to post the fastest CAGR over the next decade, fueled by growing rare‑disease awareness in Japan, South Korea, and Australia, along with increasing investment in advanced therapy manufacturing hubs in China and Singapore.
Latin America and the Middle East & Africa remain nascent markets; yet, improving diagnostic capabilities and emerging public–private partnerships suggest gradual uptake as cost‑sharing and compassionate‑use programs extend access to novel ALD treatments.
Market Drivers
Day‑to‑day demand for ALD therapeutics is driven primarily by three forces. First, the lack of effective standard therapy creates significant unmet medical need, allowing innovative treatments to command premium prices and rapid uptake among rare‑disease centers. Second, supportive regulatory frameworks—particularly the U.S. Orphan Drug Act and comparable incentives in Europe and Japan—offer market exclusivity, tax credits, and expedited review pathways that de‑risk development and boost return on investment. Third, growing advocacy from patient organizations has generated a cohesive global community that facilitates trial recruitment, disseminates screening best practices, and lobbies payers for broad access to new therapies.
Opportunities
Despite its relatively small patient base, the ALD market presents notable opportunities. Gene‑replacement and gene‑editing platforms hold potential for long‑term disease correction with single doses, positioning developers for high‑value, annuity‑type revenues through innovative payment models. Combination approaches—pairing hematopoietic stem‑cell gene therapy with metabolic modulators—could enhance efficacy in diverse phenotypes (childhood cerebral, adrenomyeloneuropathy, and Addison‑only presentations). There is also a white space for neuroprotective agents that mitigate inflammatory cascades associated with demyelination, potentially expanding the therapeutic toolkit beyond genetic correction. In parallel, digital health solutions that track cognitive decline and motor function remotely could serve as companion diagnostics, generating additional revenue streams and strengthening post‑market evidence packages.
Challenges
The market still faces significant hurdles. Complex manufacturing for autologous gene therapies demands sophisticated CMC controls and high production costs, which may limit scalability and strain payer budgets. Clinical heterogeneity in ALD progression complicates endpoint selection and increases trial‑design complexity, while the rarity of the disease makes patient recruitment challenging. Safety concerns around insertional mutagenesis for integrating vectors and immune responses to viral capsids require long‑term follow‑up and could dampen clinician confidence. Moreover, global disparities in screening infrastructure and reimbursement frameworks risk uneven patient access, particularly in low‑ and middle‑income regions.
Adrenoleukodystrophy Drugs Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 1,915.60 Million |
| Market Size in 2025 | USD 591.86 Million |
| Market Size in 2024 | USD 519.45 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 13.94% |
| Dominating Region | North Ameirca |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Treatment Type, Disease Type, Route of Administration, Distribution Channel, Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Adrenoleukodystrophy (ALD) Drugs Market Comapnies

- bluebird bio, Inc.
- Minoryx Therapeutics
- Apollo Therapeutics
- Orchard Therapeutics
- MedDay Pharmaceuticals
- SOM Biotech
- Sage Therapeutics
- Abeona Therapeutics Inc.
- Eunice Kennedy Shriver National Institute (clinical research sponsor)
- Regenxbio Inc.
- Passage Bio
- Homology Medicines
- PTC Therapeutics
- Polaryx Therapeutics
- BridgeBio Pharma
- Takeda Pharmaceutical Company Ltd.
- Sanofi S.A.
- Novartis AG
- Pfizer Inc.
- BioMarin Pharmaceutical Inc.
Segments Covered in the Report
By Treatment Type
- Gene Therapy
- Lentiviral Vector-Based Gene Therapy
- AAV-Based Gene Therapy
- Corticosteroid Replacement Therapy
- Hydrocortisone
- Prednisolone
- Hematopoietic Stem Cell Transplant (HSCT)-Linked Therapies
- Allogeneic HSCT Supportive Drugs
- Small Molecule Therapy
- Lorenzo’s Oil and Derivatives
- Other Fatty Acid Modulators
- Supportive Care & Symptomatic Therapy
- Anticonvulsants
- Anti-inflammatory Agents
By Disease Type
- Childhood Cerebral ALD (ccALD)
- Adrenomyeloneuropathy (AMN)
- Addison-Only ALD
By Route of Administration
- Oral
- Intravenous (IV)
- Intrathecal
By Distribution Channel
- Hospital Pharmacies
- Specialty Clinics
- Online Pharmacies
- Retail Pharmacies
Read Also: Alzheimers Drug Market
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344
- Arteriovenous Implants Market Enhance dialysis outcomes with advanced grafts, endovascular access, and AI-assisted precision - September 16, 2025
- Smart Retinal Implants Market Restore vision with wireless bioelectronic prosthetics and AI-powered retinal technologies - September 16, 2025
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025