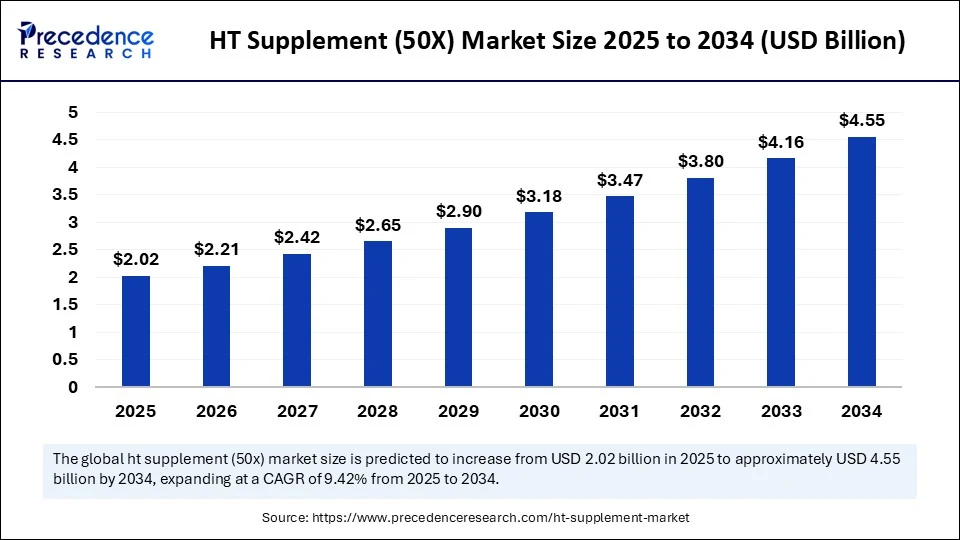
Market Key Takeaways
The HT Supplement (50X) Market in the context of laboratory and research usage is exemplified by products like Gibco™ HT Supplement (50X), a liquid mixture containing high-concentration sodium hypoxanthine and thymidine used for post-selection rescue in cell culture systems. This segment is specialized, supporting research institutions and biotech companies globally.
Demand is steady and tied to overall life sciences research activity. Major players include providers of cell culture reagents and consumables. The market thrives on technical reliability, compliance, cGMP-grade production, and supply chain consistency—especially for critical reagents.
Artificial Intelligence of Market
Within this HT Supplement (50X) Market segment, AI plays a vital role in optimizing formulation research and lab operations. AI algorithms assist in predicting optimal media additive concentrations, improving cell culture recovery and viability. Manufacturing lines for reagents use AI-controlled quality systems to ensure batch consistency and compliance under cGMP conditions.
In vendor-client operations, AI-powered procurement systems forecast lab consumable demand based on project pipelines and usage trends. Research institutions also deploy AI-driven procurement and scheduling platforms to automate ordering and avoid experiment disruptions due to stockouts.
Market Overview
The HT Supplement (50X) Market in the research context refers specifically to high-concentration media supplements—commonly, a 50X solution of sodium hypoxanthine and thymidine used in cell culture workflows. This specialized supplement is used for post-selection rescue to re-establish the nucleotide synthesis pathway in cultured cells, especially after drug selection processes.
The product must meet strict sterility, stability, and documentation standards—typically manufactured under cGMP and ISO 13485 certification. Distribution is targeted through lab supply chains, biotech service providers, and institutional procurement channels, serving research institutions, pharmaceutical development labs, and biomanufacturing facilities.
Drivers
Growth in this research-oriented HT Supplement (50X) Market is driven by:
-
Life Science Research Expansion: Increasing scale and sophistication of cell-based assays, gene editing, and therapeutic development spur demand.
-
Quality and Compliance Standards: Need for sterile, reproducible, well-characterized supplements encourages adoption of cGMP-grade products.
-
Workflow Efficiency Needs: Researchers favor ready-to-use concentrated solutions for streamlined protocols and reduced contamination risk.
-
Platformization and Commercial Adoption: Expand use of standardized media platforms by CROs and biotech companies increases demand for compatible supplements.
-
Regenerative Medicine and Gene Therapy: Advanced cell culture applications augment need for reliable high-performance additives.
Market Trends
Notable trends shaping this segment of the HT Supplement (50X) Market include:
-
cGMP & Traceability Focus: Expanded manufacturing with qualification documentation to serve clinical and GMP-compliant workflows.
-
Multiproduct Bundles: Entry of combo-media kits—including HT Supplement—with standardized inserts and protocols to ease adoption.
-
Stability Enhancements: Improved formulations aiming for extended shelf life and greater shipment resilience.
-
Digital Catalogs & E-Procurement: Shift toward streamlined online ordering with lot tracking, COA access, and automatic reordering.
-
Regional Production Redundancy: Dual or regional manufacturing sites to mitigate supply disruptions.
Opportunities
Key opportunities in this HT Supplement (50X) Market include:
-
Regional Manufacturing Hubs: Establishment of localised production in Asia or EU to reduce lead times and costs for research institutions.
-
Research Partnerships: Co-development with academic and industrial labs to tailor supplement specifications for new model systems.
-
Bundled Consumable Solutions: Selling as part of integrated cell culture kits with media and feeds represents added convenience and value.
-
Digital Protocol Integration: Embedding with digital lab notebooks and protocol platforms to recommend usage and track performance.
-
Certification Expansion: Gaining clinical-grade (GMP/ISO) registrations to support adoption in therapeutic manufacturing pipelines.
Challenges
Challenges in this HT Supplement (50X) Market segment include:
-
Supply Continuity: Lab experiments are sensitive to disruptions—consistent product availability is critical.
-
High Quality Specification Demands: Meeting cGMP and audit readiness standards increases production complexity and cost.
-
Niche Usage Volume: Usage is limited to advanced research settings—volume growth is slower compared to consumer supplement markets.
-
Competition from Generic Suppliers: Cost-sensitive labs may opt for less expensive unbranded or in-house alternatives.
-
Documentation Burden: Providing COAs, batch records, and compliance paperwork adds layers of operational overhead.
Recent Developments
Recent activity in the HT Supplement (50X) Market includes:
-
cGMP Compliance Expansion: Providers like Thermo Fisher reaffirmed supply continuity and cGMP manufacturing through 2025.
-
Facility Diversification: Dual-site production in different geographies to reduce risk and increase supply agility.
-
Process Optimization: Transition from batch to controlled continuous manufacturing to ensure concentration precision and batch consistency.
-
Supply Chain Digitization: Integration of digital lot, COA access, and inventory forecasting tools for research labs.
-
Protocol Standardization: Inclusion of HT Supplement in pre-validated media kits accelerated adoption in regulated workflows.
HT Supplement (50X) Market Companies

- Herbalife Nutrition
- Amway (Nutrilite)
- GNC Holdings, Inc.
- NOW Foods
- Nature’s Bounty
- NutraScience Labs
- Swanson Health Products
- Himalaya Herbal Healthcare
- USANA Health Sciences
- Solgar, Inc.
- Nature’s Way
- Yakult Honsha Co., Ltd.
- Garden of Life
Life Extension - Pharmavite LLC (Nature Made)
- BioTechUSA
- ON (Optimum Nutrition)
- Nutrex Hawaii
- Jarrow Formulas
- Herbaland
Also Read@ https://www.pharma-geek.com/segmented-flow-analyzer-market/
- Estrogen Replacement Therapy Market Size to Reach USD 19.46 Billion by 2034 - September 18, 2025
- Peptide Therapeutics CDMO Market Report Size, Share & Forecast 2034 - September 17, 2025
- Rare Musculoskeletal Disorder Treatments Market Size, Share & Future Trends 2034 - September 17, 2025
