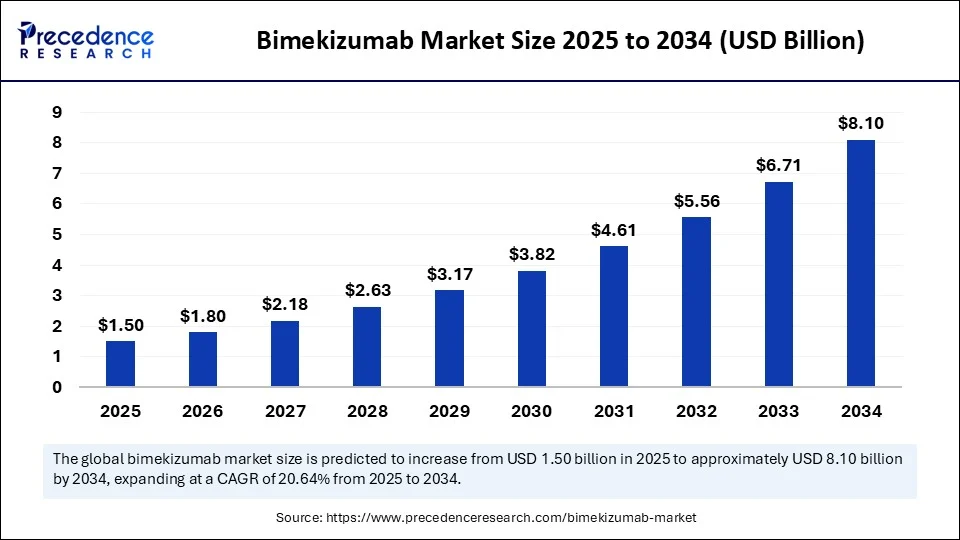
According to Precedence Research, the global bimekizumab market size is projected to reach USD 8.10 billion by 2034, growing from USD 1.24 billion in 2024, at a strong CAGR of 20.64% during the forecast period from 2025 to 2034.
The global bimekizumab (BIMZELX) market is undergoing a transformative shift as biologic therapies gain ground in the treatment of chronic autoimmune conditions. Bimekizumab, a humanized monoclonal antibody developed by UCB, offers dual inhibition of interleukin-17A and interleukin-17F—providing superior clinical outcomes in conditions such as plaque psoriasis, psoriatic arthritis, and hidradenitis suppurativa.
The demand for targeted, fast-acting biologics is surging as healthcare systems globally prioritize value-based outcomes, patient-centric treatments, and personalized medicine. Bimekizumab’s unique mechanism of action has been well-received across markets, especially in North America and Europe, where clinical trials and regulatory frameworks have paved the way for accelerated adoption.
Bimekizumab Market Key Highlights
-
Market Value in 2024: USD 1.24 Billion
-
Estimated Value by 2025: USD 1.50 Billion
-
Forecast for 2034: USD 8.10 Billion
-
CAGR (2025–2034): 20.64%
-
Top Region in 2024: North America (USD 483.6 million; 39% market share)
-
Dominant Indication: Plaque Psoriasis
-
Fastest-Growing Indication: Hidradenitis Suppurativa
-
Leading Formulation: Pre-filled syringe 160 mg/1 mL
-
Fastest-Growing Channel: Online Pharmacies
-
Major Manufacturer: UCB Pharma
AI’s Impact on the Bimekizumab Market
Artificial Intelligence is playing a transformative role in the bimekizumab market, particularly in three key areas: clinical development, patient targeting, and supply chain optimization. AI-driven platforms are being used to:
-
Identify optimal patient cohorts for clinical trials
-
Predict treatment response across genetic profiles
-
Streamline post-marketing surveillance and adverse event reporting
-
Optimize global distribution and inventory of biologic formulations
These AI-enabled capabilities are not only improving therapeutic outcomes but also reducing development costs and timelines, making bimekizumab more accessible in both developed and emerging economies.
Bimekizumab Market Key Growth Drivers
1. Surging Prevalence of Autoimmune Diseases
With over 125 million people globally affected by psoriasis and millions more by psoriatic arthritis and hidradenitis suppurativa, the demand for effective, long-lasting treatments is at an all-time high.
2. Regulatory Approvals and Label Expansion
The FDA, EMA, and other regulatory bodies have approved bimekizumab for plaque psoriasis, with clinical trials underway for other indications. Fast-track and priority review designations have supported market expansion.
3. Preference for Dual-Target Therapies
Unlike traditional IL-17A inhibitors, bimekizumab’s dual inhibition approach provides deeper and longer-lasting remission, which is reshaping treatment algorithms across dermatology and rheumatology.
4. Growing Reimbursement Support
Bimekizumab is increasingly covered under public and private insurance programs in high-income countries, further accelerating physician adoption and patient compliance.
Read Also: Wegovy Market
Bimekizumab Market Emerging Trends & Market Opportunities
Can Bimekizumab Become the Biologic Standard for Chronic Inflammation?
The market is ripe for innovation, and bimekizumab is well-positioned to be the biologic of choice across a broader spectrum of inflammatory conditions. Trends reshaping the landscape include:
-
Label Expansion into Axial Spondyloarthritis and Crohn’s Disease
-
Introduction of High-Concentration Formulations
-
Self-Administration Devices with Real-Time Monitoring
-
Teledermatology and E-Pharmacy Growth Fueling Online Channels
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 8.10 Billion |
| Market Size in 2025 | USD 1.50 Billion |
| Market Size in 2024 | USD 1.24 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 20.64% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Indication, Formulation / Presentation, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Bimekizumab Market Regional Insights
North America
Leading the market with a 39% revenue share in 2024, North America remains a strategic stronghold for bimekizumab adoption. The U.S. healthcare system’s emphasis on biologics, rapid regulatory pathways, and payer reimbursement has fueled early uptake. Moreover, partnerships with clinical research organizations and dermatology centers have enabled quick real-world validation.
Europe
Europe is a vital growth hub, with Germany, France, and the U.K. seeing broad-scale access to Bimzelx. EU countries benefit from centralized drug approvals and favorable health technology assessments (HTAs), promoting faster rollout of innovative biologics.
Asia-Pacific
This region is expected to exhibit the highest CAGR during the forecast period. Countries such as Japan, South Korea, and Australia are increasing investment in specialty drug access. China and India are also accelerating the availability of biologics through public healthcare expansion and local distribution partnerships.
Bimekizumab (BIMZELX) Market Companies

- UCB
- AbbVie (Cosentyx)
- Novartis (Cosentyx)
- Eli Lilly (Taltz)
- Janssen (Tremfya)
- Pfizer
- Amgen
- Sanofi
- Janssen (Stelara for IL23)
- Regeneron
- Boehringer Ingelheim
- Bristol-Myers Squibb
- Sandoz
- Merck
- Biogen
- Horizon Therapeutics
- MorphoSys
- Incyte
- Arcutis Biotherapeutics
- Kyowa-Kirin
Noteworthy Developments:
-
February 2025: UCB reports faster-than-expected uptake of bimekizumab in psoriatic arthritis following expanded FDA approval.
-
2024–2025: Real-world data from 5,000+ patients shows a 70%+ PASI 90 response rate sustained over 12 months.
-
Collaborations: UCB partners with AI-driven CROs for optimized clinical trial recruitment in Europe and Asia.
Other key players include AbbVie, Amgen, Novartis, and Lilly, all of whom are exploring competitive and complementary biologic pipelines.
Distribution & Formulation Insights
-
Formulation Leader: Pre-filled syringe 160 mg/1 mL held the largest revenue share in 2024.
-
Rising Trend: Pre-filled pen 320 mg/2 mL is expected to register the fastest growth, owing to ease of self-administration.
-
Distribution Channels:
-
Hospital Pharmacies dominate distribution due to controlled handling of biologics.
-
Online Pharmacies are the fastest-growing channel, boosted by e-prescriptions, chronic disease care platforms, and teleconsultations.
-
Market Challenges
Despite robust growth, the bimekizumab market faces a few key hurdles:
-
High Treatment Costs: Accessibility in low- and middle-income countries is limited.
-
Patent Cliff Risk: Market exclusivity may decline post-2030, inviting biosimilar competition.
-
Pharmacovigilance Requirements: Long-term safety monitoring adds to regulatory and operational burdens.
Case Study: Real-World Outcomes in Dermatology
UCB’s global patient registry program launched in 2024 includes over 5,000 participants across North America and Europe. The program tracks relapse rates, skin clearance, patient-reported outcomes, and adverse events. Initial results suggest bimekizumab outperforms IL-17A-only inhibitors in real-world settings, offering 12-month sustained remission in over 70% of patients.
Ready to Dive Deeper?
Discover how your organization can benefit from in-depth market insights, competitive strategies, and custom forecasts.
👉 Download the Sample Report
📅You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025
- Pill Timer Market Size to Hit $2.94 Bn by 2034 | 9.02% CAGR Growth Forecast - August 12, 2025
- Blood Glucose Monitoring Market Size to Worth USD 25.53 Billion by 2034 - August 11, 2025