Immunoprotein Diagnostic Testing Market Key Points
-
North America led the global market in 2024, accounting for the largest share of 45%.
-
Asia Pacific is projected to register the fastest CAGR of 8.74% during the forecast period.
-
By test type, the immunoglobulin diagnostic tests segment held the largest market share at 28% in 2024.
-
The C-reactive protein (CRP) diagnostic tests segment is expected to grow at the highest CAGR of 8.34% in the coming years.
-
In terms of application, the infectious disease testing segment captured the largest share of 25% in 2024.
-
The oncology testing segment is anticipated to witness the fastest CAGR of 8.15% over the forecast period.
-
By technology, the enzyme-based immunoassay segment held the dominant share of 26% in 2024.
-
The chemiluminescence assay segment is expected to expand at the fastest CAGR of 9.34% during the forecast period.
-
Based on distribution channel, the retail sales segment accounted for the highest market share of 54% in 2024.
-
The direct tender segment is forecasted to grow at the fastest CAGR of 8.14% over the projection period.
-
By end-user, hospitals and clinics represented the largest market share of 45% in 2024.
-
Diagnostic laboratories are expected to experience the fastest CAGR of 8.54% in the upcoming years.
Immunoprotein Diagnostic Testing Market Overview
The Immunoprotein Diagnostic Testing Market has emerged as a cornerstone of modern medical diagnostics, playing a pivotal role in the early detection, monitoring, and management of various diseases, particularly autoimmune disorders, infectious diseases, and cancers. Immunoproteins—such as immunoglobulins, prealbumins, haptoglobins, and C-reactive proteins—are crucial biomarkers that indicate abnormal immune responses or inflammation. Diagnostic testing based on these proteins enables clinicians to detect underlying pathologies long before the onset of severe symptoms.
This market has experienced significant growth in recent years due to the rising global burden of chronic diseases and increasing emphasis on early and preventive healthcare. Technological advances, especially in immunoassay platforms, have made testing more accurate, rapid, and accessible, both in centralized labs and near-patient settings. Furthermore, the demand for immunoprotein diagnostics surged during and after the COVID-19 pandemic, which emphasized the need for robust immune monitoring and biomarker-based diagnostics across healthcare systems. As healthcare providers transition toward personalized and precision medicine, the need for sensitive, disease-specific immunoprotein tests continues to expand.
Immunoprotein Diagnostic Testing Market Growth Factors
Several key factors are driving the robust growth of the immunoprotein diagnostic testing market. The foremost among these is the rising prevalence of autoimmune and chronic inflammatory diseases, such as rheumatoid arthritis, lupus, multiple sclerosis, and chronic kidney disease. These conditions often require regular monitoring of immune system biomarkers, for which immunoprotein testing is critical.
Another major growth factor is the increased incidence of infectious diseases, including viral, bacterial, and parasitic infections, where rapid identification of immune markers is essential for timely treatment decisions. Immunoprotein tests can help in distinguishing between types of infections and evaluating immune response severity.
Additionally, the market is benefitting from the growing geriatric population, which is more prone to immune dysfunction and chronic illnesses, thus increasing the volume of diagnostic testing. The rise of health awareness and preventive diagnostics among the younger population is also contributing to the market’s expansion.
Furthermore, advancements in immunoassay technologies, such as chemiluminescence immunoassays (CLIA), multiplex assays, and point-of-care platforms, have significantly improved testing capabilities, sensitivity, and turnaround times, facilitating broader clinical use.
Impact of AI on the Immunoprotein Diagnostic Testing Market
Artificial Intelligence (AI) is transforming the immunoprotein diagnostic testing landscape, enhancing accuracy, efficiency, and decision-making across the testing ecosystem. One of the most notable impacts of AI lies in automating diagnostic interpretation. Machine learning algorithms are increasingly being applied to immunoassay data to identify complex patterns, anomalies, and early signs of disease that might be missed by traditional analysis.
AI-powered platforms also enable real-time data analysis and predictive modeling, providing clinicians with actionable insights based on large datasets. This is particularly valuable for conditions requiring continuous immune monitoring, such as cancer immunotherapy or chronic inflammation management.
Moreover, AI is facilitating the development of smart diagnostic devices, especially in the point-of-care segment. These AI-integrated systems can perform rapid testing, interpret results instantly, and integrate findings with electronic health records for seamless clinical decision support.
AI also accelerates biomarker discovery and test development. By analyzing genomic, proteomic, and clinical datasets, AI helps identify novel immunoproteins relevant to disease pathways, thereby expanding the test portfolio and improving diagnostic precision.
Ultimately, the integration of AI in immunoprotein diagnostics enhances test throughput, reduces error rates, and supports personalized medicine approaches by correlating immune profiles with individual health trajectories.
Market Drivers
- High diagnostic demand across chronic and autoimmune diseases, necessitating regular and specific immune marker evaluation.
- Expansion of cancer immunotherapy and precision medicine, where immune system profiling, including immunoproteins, is vital for treatment planning and monitoring.
- Technological innovation in diagnostic platforms, offering faster, more sensitive, and multiplexed testing options across centralized and decentralized settings.
- Global emphasis on preventive healthcare, driving demand for early diagnostic testing, especially among aging populations.
- Increased healthcare expenditure and infrastructure development, particularly in emerging economies, which is boosting laboratory testing capabilities.
Opportunities
There are several untapped opportunities in the immunoprotein diagnostic testing market. A major opportunity lies in the development of rapid point-of-care immunoprotein tests, especially for use in rural or low-resource settings. These could expand access to critical diagnostics and support early disease intervention.
Another key opportunity is the integration of immunoprotein testing with wearable and remote diagnostic tools. Innovations in biosensors and microfluidics may soon allow real-time immunomarker monitoring outside clinical settings, which is ideal for chronic disease management and telemedicine.
Emerging markets offer substantial growth potential as governments and private healthcare providers invest in diagnostic infrastructure, improve insurance coverage, and promote public health programs targeting non-communicable diseases.
Additionally, the rising focus on personalized and precision diagnostics opens the door for highly targeted immunoprotein panels tailored to individual genetic or clinical profiles. This personalization could significantly enhance diagnostic value and therapeutic decision-making.
Challenges
Despite promising growth, the immunoprotein diagnostic testing market faces notable challenges. One of the primary concerns is the variability and complexity of immune responses between individuals, which can make interpretation of immunoprotein levels difficult without advanced analytics or contextual clinical data.
Cost constraints pose another challenge, especially in low- and middle-income countries where the affordability of sophisticated immunoassays can limit accessibility. The high cost of developing and validating new tests also hinders smaller players from entering the market.
Regulatory hurdles and lengthy approval processes for new diagnostic products can delay market entry, particularly in markets with evolving healthcare policies and infrastructure limitations.
Data privacy and integration challenges also arise as immunoprotein diagnostics become increasingly digitized and linked with AI platforms. Ensuring secure, compliant handling of sensitive health data is essential for maintaining trust and regulatory adherence.
Market Scope
| Report Coverage | Details |
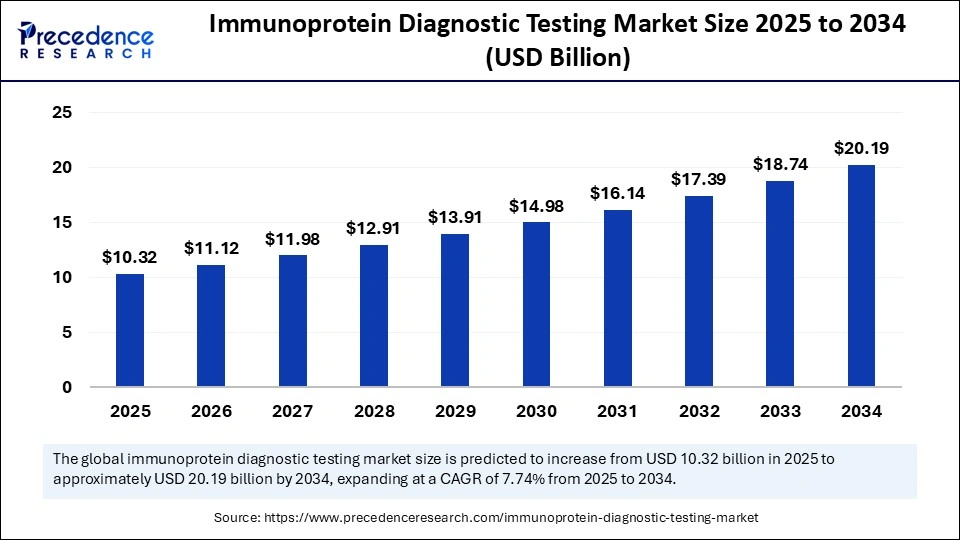
| Market Size by 2034 | USD 20.19 Billion |
| Market Size in 2025 | USD 10.32 Billion |
| Market Size in 2024 | USD 9.58 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 7.74% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Test, Application, Technology, Distribution Channel, End-User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Immunoprotein Diagnostic Testing Market Regional Outlook
North America currently dominates the global immunoprotein diagnostic testing market due to its advanced healthcare infrastructure, robust research activities, and early adoption of new diagnostic technologies. The presence of leading biotech and diagnostic companies, along with favorable reimbursement frameworks, also supports market leadership in the U.S. and Canada.
Europe follows closely, with strong contributions from countries like Germany, the UK, and France. The region benefits from a well-established public healthcare system and growing investments in chronic disease prevention and biomarker research.
Asia Pacific is the fastest-growing regional market, fueled by increasing healthcare expenditure, rising chronic disease burden, and expanding diagnostic laboratory networks in countries like China, India, and Japan. Government support for healthcare modernization and a growing middle-class population with rising health awareness are further accelerating growth in this region.
Latin America and the Middle East & Africa are emerging markets that show promising potential due to improving healthcare access, growth in medical tourism, and rising investments in diagnostic technologies. However, challenges related to regulatory harmonization and cost may impact rapid adoption.
Immunoprotein Diagnostic Testing Market Companies

- Agilent Technologies, Inc.
- Danaher Corporation
- Thermo Fisher Scientific Inc.
- PerkinElmer Inc.
- Bio-Rad Laboratories, Inc.
- Aurora Biomed Inc.
- Tecan Trading AG
- Promega Corporation
- Charles River Laboratories
- Creative Biolabs
Segments Covered in the Report
By Test
- Complement System Proteins Diagnostic Tests
- Free Light Chain Diagnostic Tests
- Haptoglobin Diagnostic Tests
- Immunoglobulin Diagnostic Tests
- Prealbumin Diagnostic Tests
- C-Reactive Protein (CRP) Diagnostic Tests
By Application
- Infectious Disease Testing
- Oncology Testing
- Endocrine Testing
- Toxicology Testing
- Allergy Testing
- Autoimmune Disease Testing
By Technology
- Radioimmunoassay
- Enzyme-Based Immunoassay
- Chemiluminescence Assay
- Immunofluorescence Assay
- Immunoturbidity Assay
- Immunoprotein Electrophoresis
By Distribution Channel
- Direct Tender
- Retail Sales
By End-User
- Hospitals and Clinics
- Diagnostic Laboratories
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Read Also: Human PBMC Isolation Kit Market
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6259
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344
- Arteriovenous Implants Market Enhance dialysis outcomes with advanced grafts, endovascular access, and AI-assisted precision - September 16, 2025
- Smart Retinal Implants Market Restore vision with wireless bioelectronic prosthetics and AI-powered retinal technologies - September 16, 2025
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025
