-
North America led the global human PBMC isolation kit market with the highest share of 41% in 2024.
-
Asia Pacific is projected to register the fastest CAGR in the market between 2025 and 2034.
-
By product type, the density gradient medium segment held the largest market share in 2024.
-
The cell separation columns segment, by product type, is expected to grow at the highest CAGR over the forecast period.
-
Based on the source of PBMCs, the whole blood segment contributed the largest market share in 2024.
-
The leukapheresis products segment is expected to experience the fastest growth during the projected period.
-
In terms of application, the clinical research segment dominated the market in 2024.
-
The pharmaceutical development segment is anticipated to grow at the fastest rate over the forecast timeline.
-
Among end users, academic and research institutes held the largest market share in 2024.
-
Biotechnology companies are expected to witness the fastest CAGR among end users during the forecast period.
-
By technology, the density gradient centrifugation segment accounted for the highest market share in 2024.
-
The magnetic bead-based separation segment is expected to grow at a remarkable CAGR from 2025 to 2034..
Human PBMC Isolation Kit Market Overview
The Human PBMC Isolation Kit Market is a vital segment within the broader life sciences and biomedical research industries. Peripheral Blood Mononuclear Cells (PBMCs), which include lymphocytes (T cells, B cells, and NK cells) and monocytes, are essential for a wide range of immunological and pathological studies. PBMCs are commonly used in immunotherapy research, vaccine development, infectious disease monitoring, cancer immunology, and drug discovery. The isolation of high-quality, viable PBMCs is critical for generating reliable and reproducible results, and this demand has led to increased adoption of standardized isolation kits.
Human PBMC isolation kits are designed to provide simplified, reproducible, and efficient separation of mononuclear cells from whole blood samples using density gradient centrifugation or other novel techniques. These kits are widely used in academic institutions, biopharma companies, contract research organizations (CROs), and clinical laboratories. The market is experiencing steady growth due to the expansion of immunological research and a surge in the demand for cell-based assays and personalized medicine. Post-pandemic, research into immune responses and vaccine efficacy has intensified globally, further driving demand for high-performance PBMC isolation tools.
Human PBMC Isolation Kit Market Growth Factors
Several key factors are fueling the growth of the Human PBMC Isolation Kit Market. Foremost is the increased focus on immunotherapy and immunological research, particularly in oncology, autoimmune disorders, and infectious diseases. As therapies become more personalized, PBMCs are often used to evaluate immune responses to specific treatments, requiring consistent and high-yield cell isolation techniques.
The growing trend in precision medicine and cell-based diagnostics is another significant growth driver. PBMCs are valuable for identifying biomarkers, studying gene expression, and conducting cytotoxicity and cytokine release assays, all of which are central to personalized therapeutic approaches.
Additionally, the proliferation of clinical trials and translational research involving immune cells has contributed to the rising demand for user-friendly and reliable PBMC isolation kits. These kits save time and ensure quality control, making them essential tools for high-throughput research environments.
The market is also being driven by the increased prevalence of chronic conditions such as cancer, HIV, and autoimmune diseases, which require frequent immune profiling and biomarker studies using PBMCs. The rise in academic-industry collaborations and biopharma R&D spending further supports market expansion.
Impact of AI on the Human PBMC Isolation Kit Market
Artificial Intelligence (AI) is beginning to make a transformative impact on the Human PBMC Isolation Kit Market, primarily through process optimization, automation, and data integration. While AI does not directly isolate PBMCs, it plays a crucial role in enhancing the efficiency, scalability, and precision of cell isolation workflows and subsequent analysis.
AI-powered laboratory automation systems are being integrated into PBMC isolation workflows to reduce manual errors, standardize protocols, and increase throughput. These smart systems can adjust centrifugation speeds, monitor reagent usage, and maintain consistency across multiple samples, improving both the yield and viability of isolated PBMCs.
In addition, AI is enabling advanced downstream analysis of isolated PBMCs. Machine learning algorithms are used to analyze flow cytometry data, identify cellular subtypes, and interpret functional assays with greater accuracy and speed. AI also enhances the reproducibility of results by identifying patterns in complex datasets that may not be evident through traditional analysis.
Furthermore, AI-driven bioinformatics platforms facilitate real-time data correlation between PBMC characteristics and clinical outcomes, supporting drug development and biomarker discovery. In this way, AI enhances the value proposition of PBMC isolation kits by linking cell separation processes with actionable biological insights.
Market Drivers
Expansion of immunology and oncology research, which relies heavily on immune cell analysis and PBMC-derived assays for understanding disease mechanisms and evaluating therapeutics.
Growth in vaccine development and infectious disease monitoring, where PBMCs are used to assess immune responses, particularly in viral infections like HIV, COVID-19, and hepatitis.
Increased adoption of cell-based diagnostics, which require consistent and high-quality PBMC preparations for reliable test results.
Demand for standardized, ready-to-use kits in both research and clinical laboratories, reducing the dependency on in-house manual protocols and increasing the reproducibility of experiments.
Rising biopharmaceutical investment and government funding in life science research, encouraging greater use of PBMC-based studies across multiple therapeutic areas.
Opportunities
The market presents significant opportunities for innovation and expansion. One of the most promising areas is the development of automated PBMC isolation systems that integrate with existing lab hardware and reduce technician workload. Automation will be particularly valuable for high-throughput research and clinical trial environments.
There is also growing demand for kits compatible with frozen or cryopreserved blood samples, which are essential for longitudinal studies and global research collaborations where sample transport is involved.
Additionally, expanding into emerging markets with growing biotech ecosystems—such as Southeast Asia, the Middle East, and Latin America—presents untapped potential for manufacturers. Localized manufacturing and distribution strategies can help penetrate these regions effectively.
Opportunities also exist in the customization of kits for specific research applications, such as T-cell enrichment or monocyte isolation, and in the development of kits optimized for pediatric or low-volume samples, which are in high demand in clinical research.
Challenges
Despite its promising growth, the market faces several challenges. Variability in blood samples and donor-specific factors can affect the yield and purity of PBMCs, making standardization difficult across studies. Addressing this requires further innovation in kit chemistry and protocols.
Another challenge is the high cost of high-quality isolation kits, which can limit accessibility in cost-sensitive academic and clinical settings, especially in developing countries.
There is also increased competition from alternative cell isolation technologies, such as magnetic bead-based separation and microfluidics, which offer potentially faster or more selective isolation of certain cell types.
Moreover, regulatory and ethical constraints around the use of human blood samples, especially in multicenter trials, can complicate logistics, requiring strict adherence to compliance and quality assurance protocols.
Market Scope
| Report Coverage | Details |
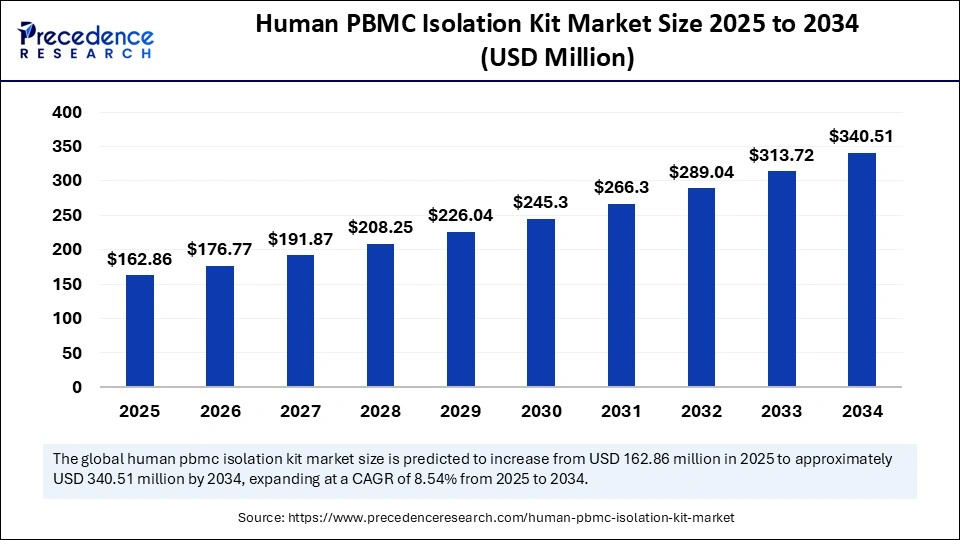
| Market Size by 2034 | USD 340.51 Million |
| Market Size in 2025 | USD 162.86 Million |
| Market Size in 2024 | USD 150.05 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.54% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Deployoment, Technology, End User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Regional Outlook
North America currently leads the Human PBMC Isolation Kit Market, driven by its advanced biomedical research infrastructure, high R&D investment by biotech and pharma companies, and the presence of key market players. The United States, in particular, benefits from a strong network of academic research centers, CROs, and life science incubators.
Europe follows closely, with countries like Germany, the UK, and France at the forefront of immunology and translational medicine. European Union funding programs and collaborative research frameworks further bolster PBMC kit usage in clinical studies and academic research.
Asia Pacific is expected to register the fastest growth rate, propelled by rising investments in biopharmaceutical R&D, increasing prevalence of chronic diseases, and growing government support for healthcare innovation. China, India, South Korea, and Japan are becoming key hubs for clinical research and CRO activity, making them lucrative markets for PBMC kits.
Latin America, the Middle East, and Africa are emerging markets where increased medical research infrastructure, disease surveillance efforts, and global health initiatives are creating new opportunities for adoption, though growth may be hindered by limited funding and regulatory complexity.
Human PBMC Isolation Kit Market Companies

- Abcam
- Amsbio
- BioVision
- Miltenyi Biotec
- Stemcell Technologies
- Thermo Fisher Scientific
- Fabgennix International Inc
- Zen-Bio
Segments Covered in the Report
By Product Type
- Cell Separation Columns
- Density Gradient Medium
- Separation Kits (Magnetic, Non-magnetic)
- Filtration Devices
By Source of PBMCs
- Buffy Coat
- Bone Marrow
- Whole Blood
- Leukapheresis Products
By Application
- Diagnostics
- Clinical Research
- Pharmaceutical Development
- Stem Cell Research
- Immunology Studies
By End User
- Academic & Research Institutes
- Biotechnology Companies
- Clinical Laboratories
- Pharmaceutical Companies
By Technology
- Affinity Chromatography
- Density Gradient Centrifugation
- Filtration Techniques
- Magnetic bead-based Separation
By Region
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- Latin America
Read Also: Heart Valve Devices Market
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6258
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344
- Arteriovenous Implants Market Enhance dialysis outcomes with advanced grafts, endovascular access, and AI-assisted precision - September 16, 2025
- Smart Retinal Implants Market Restore vision with wireless bioelectronic prosthetics and AI-powered retinal technologies - September 16, 2025
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025
